Figure 4.
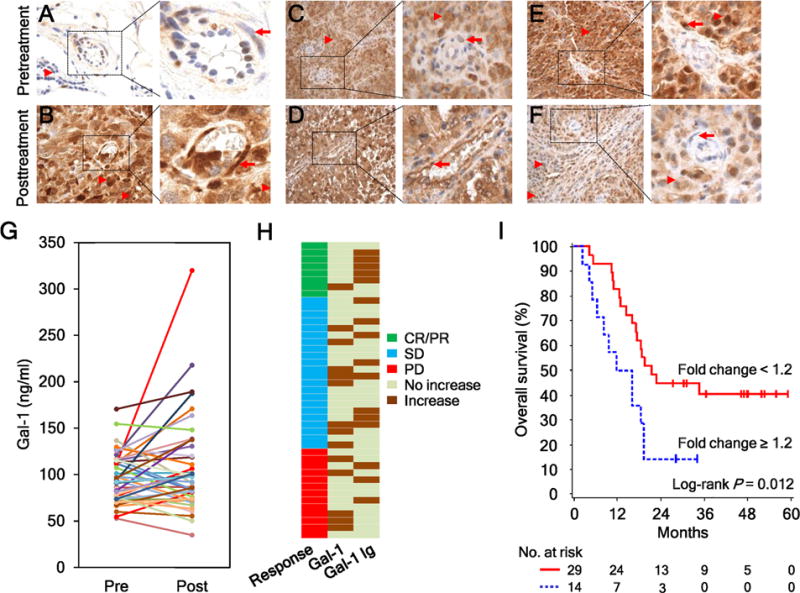
Ipi-Bev therapy altered Gal-1 expression in tumors and in the circulation. A and B, Patient P27. Gal-1 expression was hardly detected in the pretreatment tumors (A) but was intensive in tumor cells and tumor associated endothelial cells (TEC) posttreatment (B). Representative TEC and tumor cells are indicated with arrows and arrow heads respectively. C and D, Patient P20. Gal-1 expression was highly expressed in tumor cells but not TEC pretreatment (C). Strong Gal-1 expression was observed in tumor cells and TEC posttreatment (D). E and F, Patient P28. Gal-1 was highly expressed in tumor cells and TEC pretreatment (E). Reduced and no Gal-1 expression was detected in tumor cells and TEC posttreatment (F). G, Pretreatment and posttreatment circulating Gal-1 concentrations of the patients (n = 43). H, Different subsets of patients had increased circulating Gal-1 and antibody responses to Gal-1 in response to Ipi-Bev. Patients are grouped by clinical responses. I, Kaplan-Meier survival curves of patients based on circulating Gal-1 fold changes as a result of Ipi-Bev treatment. Increases in circulating Gal-1 were significantly associated with shortened overall survival (P = 0.012). The median survival of the patients with circulating Gal-1fold change ≥ 1.2 was 13.9 months (95% CI, 5–19), while that of patients with fold change < 1.2 was 21.6 months (95% CI, 16 to ∞).
