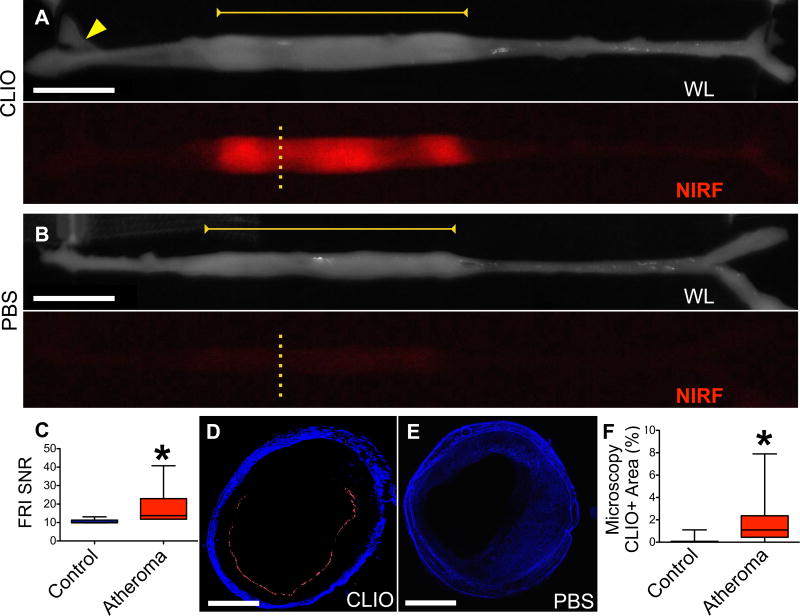
Figure 1. Ex vivo assessment of NIRF nanoparticle deposition in atheroma.
(A) Representative ex vivo fluorescence reflectance imaging (FRI) in a rabbit 24 hours after CLIO-CyAm7 injection. White light (WL) image shows the resected aorta, stretched to premortem length determined by in vivo x-ray angiography. CLIO-CyAm7 signal evolved in atheroma (yellow bar) in contrast to minimal signal in the uninjured aorta, iliac bifurcation, or renal artery (yellow arrow). (B) Ex vivo FRI of a rabbit injected with PBS as a control exhibits minimal NIRF signal in atheroma (yellow bar). (C) Quantification of FRI signal in untriggered animals (n=6) showing significantly higher CLIO-CyAm7 signal in regions of atheroma compared to control renal artery (p=0.03). (D) Corresponding fluorescence microscopy (FM, yellow dashed line Figure 1A) of CLIO-CyAm7-injected atheroma showing luminal surface CLIO-CyAm7 signal (red) non-circumferentially distributed. (E) FM of a control atheroma (PBS injection, yellow dashed line Figure 1B) showing minimal CLIO-CyAm7 signal despite substantial atheroma detectable by autofluorescence. (F) FM analysis of percent positive CLIO-CyAm7 pixels in experimental, triggered rabbits at 48 hours showing increased CLIO-CyAm7 accumulation in regions of atheroma versus uninjured aorta without evidence of intimal thickening (p<0.0001, n=183 slices). Scale bars; FRI = 1cm, Microscopy = 1mm. CLIO-CyAm7 = red, Autofluorescence = blue.