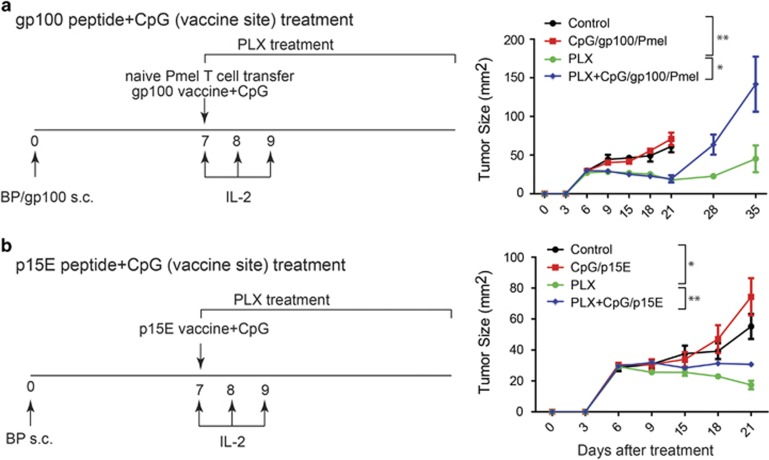
Figure 1.
BRAF inhibitor-induced antitumor responses are impaired when combined with CpG-based peptide vaccines. (a) A previously established murine melanoma cell line bearing BRAF and PTEN mutation (BP) was provided by Dr Wargo (MD Anderson Cancer Center)3 and maintained in RPMI 1640 with 10% FCS and 100 μg/ml Normocin (Invivogen, San Diego, CA, USA). To generate gp100-expressing murine cell line (BP/gp100), BP cells were transduced with the lentiviral vector encoding full-length human gp100 as previously described.34 Tumor growth was induced by subcutaneous injection of 5 × 105 BP/gp100 cells in 6- to 8-week-old female C57BL/6 mice (from Charles River NCI, Frederick, MD, USA). Seven days after tumor challenge, tumor-bearing mice were treated with a selective BRAF inhibitor PLX4720 (Plexxikon, Berkeley, CA, USA; 100 mg/kg in 3% DMSO and 1% methylcellulose by oral gavage daily35), tumor vaccine with 50 μg CpG (CpG-ODN-2216 synthesized by Invitrogen Life Technologies (Carlsbad, CA, USA) and injected intratumorally once per week for 3 weeks) and 100 μg synthetic, high-affinity H-2Db-restricted hgp10025–33 peptide (KVPRNQDWL, purchased from Peptides International (Louisville, KY, USA) at a purity >95% and injected subcutaneously), or both. For groups containing the tumor vaccine, 1 × 103 Pmel-1T cells (isolated from Pmel-1 TCR/Thy1.1 mice from in-house breeding colonies) were intravenously administered and 100 μg anti-CD40 (Bioxcell, West Lebanon, NH, USA) was intraperitoneally injected on day 7, and 1 × 105 IU rhIL-2 protein (Prometheus Laboratories Inc, San Diego, CA, USA) were intraperitoneally injected on days 7, 8 and 9. (b) Tumor growth was induced by subcutaneous injection of 5 × 105 BP cells in 6- to 8-week-old female C57BL/6 mice. Seven days after tumor challenge, tumor-bearing mice were treated with PLX4720, tumor vaccine with 50 μg CpG and 100 μg H-2Kb-restricted mouse P15E134–141 peptide (KSPWFTTL, purchased from Peptides International at a purity >95% and injected subcutaneously), or both. For groups containing the tumor vaccine, 100 μg anti-CD40 was intraperitoneally injected on day 7, and 1 × 105 IU rhIL-2 protein were intraperitoneally injected on days 7, 8 and 9. Tumor-bearing mice treated with vehicle (3% DMSO and 1% methylcellulose) were used as control. Tumor growth was monitored every 3 days by measuring the perpendicular diameters of tumors. N=5 mice per group. Data expressed as mean±s.e.m. *P<0.05, **P<0.01, two-way ANOVA plus post hoc Turkey test.