Figure 4.
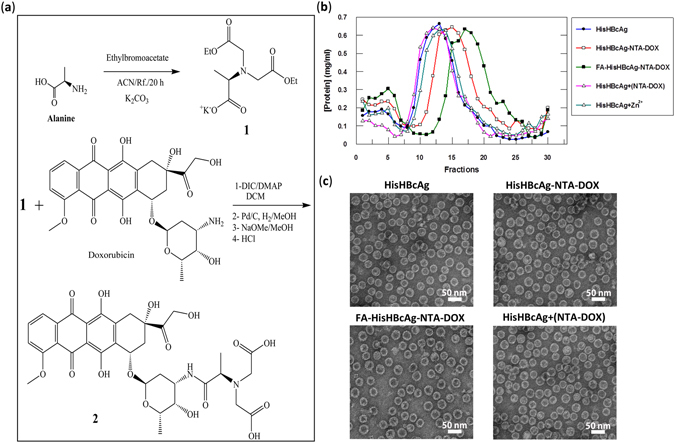
Synthesis and immobilisation of NTA-DOX on His-tagged VLNPs. (a) Synthesis of NTA-DOX. The amine group of alanine (Ala) was fully protected with ethylbromoacetate using K2CO3 under reflux condition to obtain diester-Ala (compound 1). Then, the free carboxylic acid of diester-Ala was activated by N,N′-diisopropylcarbodiimide (DIC) and 4-Dimethylaminopyridine (DMAP). The activated diester-Ala was reacted with doxorubicin (DOX) to produce diester-Ala-DOX, which was then converted to dicarboxylic-Ala-DOX (NTA-DOX; compound 2) by the hydrolysis method using NaOMe in methanol. (b) The NTA-DOX was incubated with the HisHBcAg nanoparticles (HisHBcAg) and folic acid (FA)-conjugated HisHBcAg nanoparticles (FA-HisHBcAg) in the presence of Zn2+. The nanoparticles conjugated with NTA-DOX were purified by sucrose density gradient ultracentrifugation. The protein amount in each fraction (400 μL) was determined using the Bradford assay. The HisHBcAg nanoparticles (HisHBcAg), HisHBcAg nanoparticles incubated with NTA-DOX in the absence of Zn2+ [HisHBcAg + (NTA-DOX)] and HisHBcAg nanoparticles incubated with Zn2+ (HisHBcAg + Zn2+) served as negative controls. (c) Electron micrographs of different HisHBcAg VLNPs formed by HisHBcAg, HisHBcAg-NTA-DOX, FA-HisHBcAg-NTA-DOX, and HisHBcAg + (NTA-DOX). White bars indicate 50 nm.
