Figure 1.
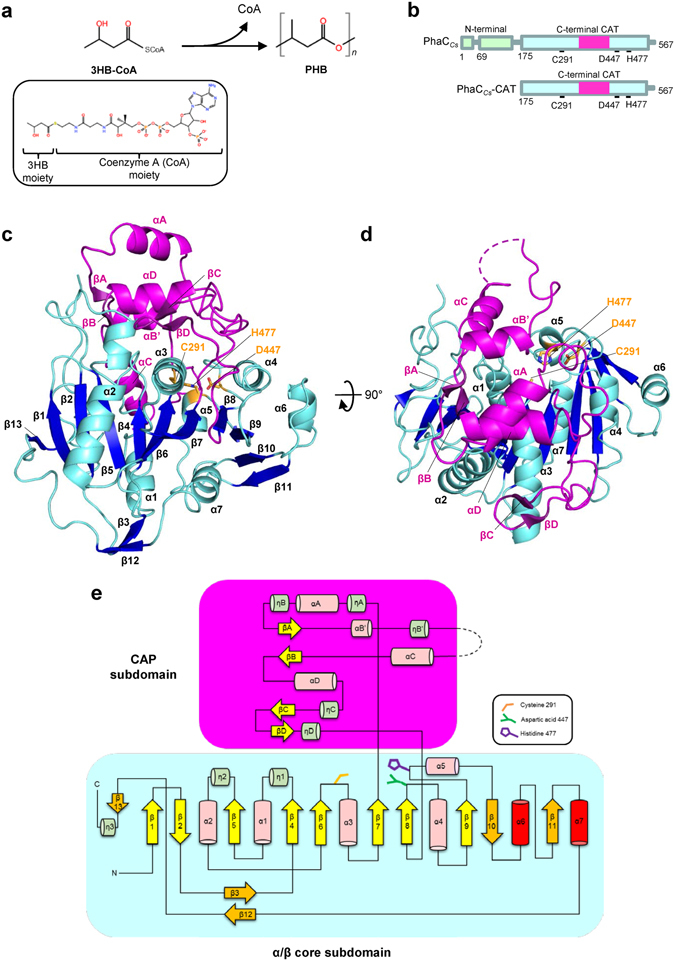
Structure of PhaCCs-CAT. (a) Polymerization reaction catalyzed by PhaC. Polymerization of the substrate, 3-hydroxybutyryl coenzyme A (3HB-CoA), into poly 3-hydroxylbutyrate (PHB) is catalyzed by PhaC with release of CoA. The chemical structure of 3HB-CoA is shown in the inset. (b) Domain organization of PhaCCs. PhaCCs consists of two domains, the N-terminal domain which is important for stabilizing dimeric PhaC and the C-terminal catalytic (CAT) domain containing conserved active site including triad residues Cys, His and Asp. (c) A side view of PhaCCs-CAT. The catalytic domain of PhaCCs comprises the α/β core subdomain (residues 175–318, 439–562 in cyan) and the CAP subdomain (residues 319–438 in violet). The side chains of the catalytic triad (Cys291, His477 and Asp447) are shown in orange. (d) As in b, but a top view of PhaCCs-CAT. The active site is covered by the CAP subdomain. (e) Schematic presentation of the secondary structure topology of PhaCCs-CAT (mol B). The CAT domain contains the CAP and core subdomains. The core subdomain comprises 13 strands and 10 helices. Nucleophilic Cys291 is located between β6 and α3, conserved His477 is located between β9 and α5, and Asp447 is located between β8 and α4. The CAP subdomain is connected from β7 and back to the core domain through β8. The catalytic triad is covered by the CAP subdomain which blocks the substrate entry pathway. The other strands (orange) and helices (red) shown represent additional secondary structures observed when comparing the canonical α/β hydrolase fold.
