Figure 6.
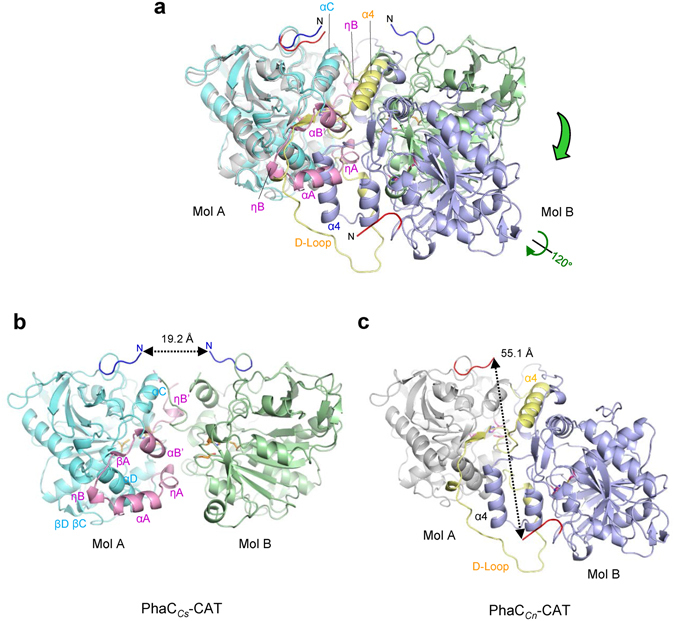
Conformational changes in the CAP subdomain induce dimer organization as revealed by comparison of PhaCCs-CAT and PhaCCn-CAT dimers. (a) Overlay of the PhaCCs-CAT dimer (cyan and green) on the PhaCCn-CAT dimer (gray and purple). One protomer (cyan) of the PhaCCs-CAT dimer is superimposed on one protomer (gray) of the PhaCCn-CAT dimer. The distance between the catalytic cysteine residues in the PhaCCs-CAT dimer is 28.1 Å, while that in the PhaCCn-CAT dimer is 33.3 Å. (b) The PhaCCs-CAT dimer. The distance between the N-termini is 19.2 Å. (c) The PhaCCn-CAT dimer with mol A in the same orientation as mol A in the PhaCCs-CAT dimer as in (b). The dimer interface is reorganized by refolding/unfolding of the LID region with accompanying rotation of one protomer. The distance between the N-termini is 55.1 Å.
