Figure 8.
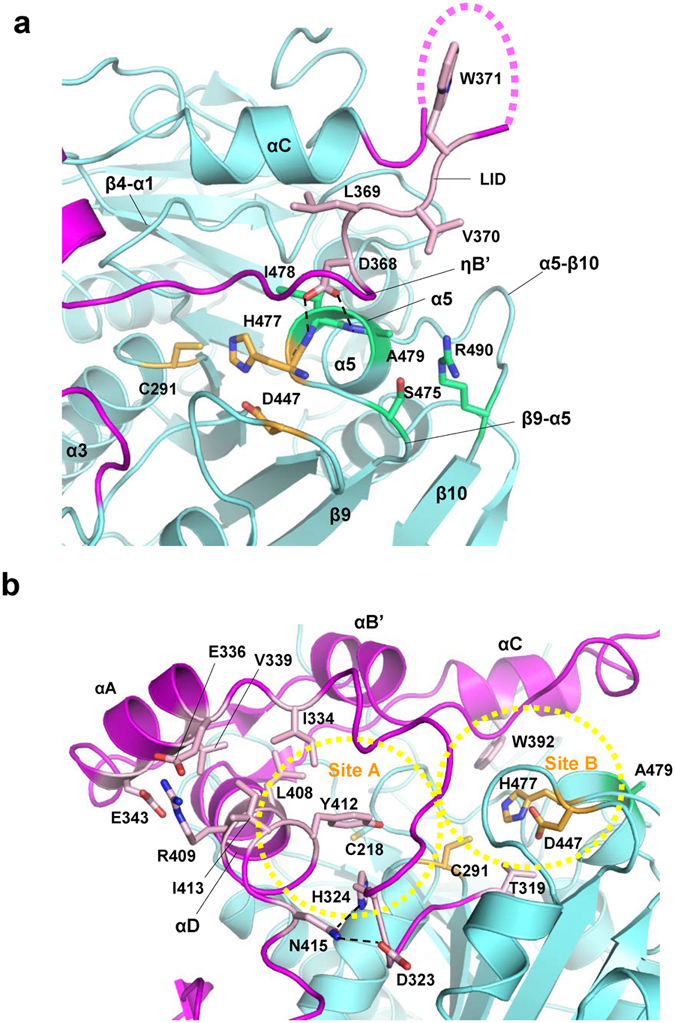
The channel of the active site of PhaCCs. (a) A close-up view of the β9-α5-β10 segment forming part of the active site of PhaCCs-CAT. Ala479 of PhaCCs is located at α5 helix, where His477 of the catalytic triad is also located. Asp368 of the LID region stabilizes α5 helix by forming hydrogen bonds to the main chain amide groups of Ile478 and Ala479 residues which form the helix. Ala479 corresponds to Ala510 of PhaCCn. (b) A view of the channel at the active site of PhaCCs-CAT. Trp392 of PhaCCs is located in the αC helix of the CAP subdomain and faces Site B of the channel where a water cluster is present. Tyr412 and Ile413 are located in the αD helix of the CAP subdomain. Tyr412 projects into Site A of the channel, while Ile413 forms a hydrophobic core with other aliphatic residues.
