Abstract
Objective
High levels of high-frequency heart-rate variability (HF-HRV), related to parasympathetic-nervous-system functioning, have been associated with longer survival in patients with myocardial infarction, acute trauma, and in patients undergoing palliative care. From animal studies linking higher vagal activity with better immune-system functioning and reduced metastases, we hypothesized that higher HF-HRV would predict longer survival in patients with metastatic or recurrent breast cancer (MRBC).
Methods
Eighty-seven patients with MRBC participated in a laboratory task including a 5-minute resting-baseline ECG. HF-HRV was computed as the natural logarithm of the summed power spectral density of RR intervals (0.15--0.50 Hz). In this secondary analysis, of a study testing whether diurnal cortisol slope predicted survival, we tested the association between resting baseline HF-HRV on survival using Cox Proportional Hazards models.
Results
A total of 50 patients died during a median follow-up of 7.99 years. Higher baseline HF-HRV predicted significantly longer survival, with a hazard ratio=0.75 (95% confidence interval=0.60 to 0.92; p=.006). Visceral metastasis status and baseline HR were related to both HF-HRV and survival. However, a combination of HF-HRV and HR further improved survival prediction, with a hazard ratio of 0.64 (95% CI=0.48 to 0.85; p=.002).
Conclusions
Vagal activity of patients with MRBC strongly predicted their survival, extending the known predictive window of HF-HRV in cancer beyond palliative care. Vagal activity can be altered by behavioral, pharmacological, and surgical interventions and may be a promising target for extending life expectancy in patients suffering from metastasizing cancer.
Keywords: Heart Rate Variability, Respiratory Sinus Arrhythmia, Parasympathetic Nervous System, Immune System, Cancer Survival, Breast Cancer
Introduction
Until recently, researchers studying cancer patients’ psychosocial morbidity or mortality rarely examined autonomic dysregulation(1, 2). However, accumulating evidence, mainly from animal research, suggests that autonomic pathways connecting the central nervous system (CNS) with the periphery may play a role in the clinical course of cancer. Tumors often develop in conjunction with chronic inflammation and an inflammation-modulating vagal-feedback circuit from the nucleus tractus solitarius to the periphery has been proposed(3). It consists of 1) an afferent path sensing proinflammatory cytokine activity and informing the brain about tumorigenesis(3–6), and 2) an efferent path subsequently increasing activity and releasing acetylcholine that spurs local anti-inflammatory action by attenuating proinflammatory cytokine activity(6–8). In summary, based on recent evidence, the vagus nerve is likely involved in inflammatory regulation of tumor processes(3–7,9–13).
The high-frequency spectral component of heart-rate variability (HF-HRV), an established index of vagal functioning, has gained prominence in clinical research because of its association with mental and physical disorders(14, 15). The neurovisceral-integration model emphasizes the role of the vagal system in regulating allostasis and posits that lowered HF-HRV is associated with poor health across a variety of diseases because it reflects excess allostatic load(15). HF-HRV has also been linked to increased mortality: for example, lower HF-HRV significantly predicted shorter survival following myocardial infarction in a number of clinical and epidemiological studies(16–18). Low HF-HRV and related HRV metrics (e.g., standard deviations of normal-to-normal R-R intervals–SDNN), predicted mortality in trauma studies including in over 2000 trauma patients, regardless of anatomic location or severity of injury(19).
Though several breast cancer studies document associations between HF-HRV and depression and anxiety(1, 20, 21) and treatment adherence(22), none link it with MRBC survival. In other cancer studies, lower HF-HRV predicted shorter survival for hepatocellular carcinoma hospice patients(23), and lower SDNN (and higher heart-rate (HR)) also predicted shorter survival in hospice patients(24). Lower SDNN and/or HF-HRV also correlated with a higher colon-cancer marker carcinoembryonic antigen(25), predicted prostate cancer death(26), and predicted shorter all-cause and cancer survival in an epidemiological study of middle-aged men(27).
This evidence suggests that a measure of vagal functioning like HF-HRV may predict long-term cancer survival. In the current study, we assessed HF-HRV at baseline in a sample of 87 patients with MRBC and tested this survival hypothesis once mortality was above 50% (over the subsequent 7–8 years). These data represent a secondary analysis from a study powered to test the hypothesis that the diurnal slope of cortisol would predict survival in this sample(28) and to examine stress responses in metastatic or recurrent breast cancer patients(1). We hypothesized that higher initial HF-HRV would be associated with longer survival. If analysis confirmed this hypothesis, it could have clinical implications: HF-HRV may help identify patients at risk of early mortality in need of special care(24). More importantly, it may point to mechanisms of disease progression that clinicians could target with behavioral, pharmacological, or surgical interventions that may increase vagal activity(29–36).
Methods
Participants
The Stanford Institutional Review Board approved all study materials insuring they conformed to HIPAA regulations. Referrals came from Stanford and Bay-Area oncologists, responses to newspaper advertisements, and word-of-mouth. Women could earn up to $500 if they were able to complete the protocol (see(1, 37)).
From 221 women with MRBC recruited from 2002–2004, 29 were ineligible, 83 not interested, and two died before the study began. Of the remaining 107 who provided written informed consent, we determined that four patients were not eligible because of medical reasons (e.g. second primary cancer rather than MRBC). Of the 103, ten dropped out before HF-HRV assessment and we excluded 6 physiological recordings because of artifacts or technical difficulties, leaving 87 patients for analyses.
Inclusion criteria were metastatic or recurrent breast cancer; Karnofsky rating of at least 70%; residence within the Greater San Francisco Bay Area; and proficiency in English. Exclusion criteria were other active cancers within ten years (excepting basal cell or squamous cell carcinomas of the skin, or in situ cervical cancer), positive supraclavicular lymph nodes as the only metastatic lesion, a concurrent medical condition influencing short-term survival, using a corticosteroid within the month, diabetes, taking benzodiazepines, or history of major psychiatric illness requiring hospitalization or medication (except depression or anxiety).
Procedure
Patients with MRBC participated in the Trier Social Stress Task (TSST)(38), a standardized social/cognitive stressor, during which we collected 5-min of autonomic data for these analyses from a 5-min pre-stress resting baseline in upright seated position. Most studies testing vagal activity as a predictor of mortality used resting baseline values, representing a stable autonomic trait and thus chronic allostatic load. We previously reported significant associations between HF-HRV and depression(1) and sleep efficiency(36) in this sample.
Measures
Demographic and Medical Variables
Participants self-reported demographic variables (age, ethnicity, education, income) and both patients and their physicians reported on medical variables (disease-free interval, estrogen receptor status, site of metastasis/recurrence, types of treatment).
Cardiovascular Physiological Measurements and Data Reduction
Experimenters obtained a standard Lead-II ECG sampled at 400 Hz. Placement of ECG electrodes, data recording, and reduction followed conventions and published guidelines(14). We assessed HF-HRV as heart-rate variability within the high-frequency band associated with respiration(14). Following established guidelines(39), we used 0.15–0.50 Hz frequency. We computed 5-min resting-baseline HF-HRV and mean HR, edited, and exported using ANSLAB Software (Society for Psychophysiological Research software repository, http://www.sprweb.org)(1, 40, 41). To compute HF-HRV, we edited heart-period values computed from Lead-II ECG signals for outliers from ectopic beats or artifact and converted them into time-series with 4 Hz resolution using cubic-spline interpolation. We linearly de-trended these, and computed power-spectral densities for each baseline period using the Welch algorithm(42), creating ensemble averages of successive periodograms. We derived averages from spectra estimated for 60-s segments, overlapping by half. For each 60-s segment, we analyzed 256 points, which includes 240 sampled points with zero padding. The segments were Hanning-windowed and subjected to fast Fourier transform. We adjusted estimates of power to account for attenuation produced by the Hanning window and normalized distributions with natural-logarithm transformation. Although respiratory variables might influence associations between vagal activity and HF-HRV (e.g.,(43)), we did not adjust for respiration, less useful in individual-difference data: individuals differ in respiratory function due to factors unrelated to vagal activity like lung size, basal metabolic rate, and respiratory pacemaker function(43).
All-Cause Mortality and Survival Data
We collected survival data from the Social Security Death Index, medical records, and family reports. This is an all-cause survival analysis; however, for all deaths reported, the immediate, underlying, or secondary cause was cancer, breast cancer, or MRBC.
Possible Confounding Measures
We assessed the role of physical activity, depression, stress, fatigue and sleep efficiency as possible confounding factors (proxies). Participants completed the Seven-Day Physical Activity Recall which classifies activities into levels by energy requirements and a calculation of metabolic units per day (METs)(44, 45), a ratio of working/resting metabolic rate: Sleep=1, light activity=1.0–2.9, moderate activity=3.0–5.0, hard activity=5.1–6.9, and very hard activity=7.0+. We aggregated METs by week and divided by seven for METs/day(46). Due to our previous finding of an association between depression and HF-HRV(1) we utilized both a continuous measure, the Beck Depression Inventory (BDI)(47), and our dichotomous measure(1). In the dichotomous measure(1), the depressed group included those with current SCID diagnoses (n=17), and women currently taking antidepressants except those prescribed primarily for sleep (n = 28). We used the Perceived Stress Scale--short form (PSS)(48) to measure previous-month perception of stress (10, 5-point Likert-type items 0=never to 4=very often). We used the MOS-SF 36 Health Survey(49) to assess fatigue by using the reversed and standardized vitality score.
Due to a previous significant finding in these data(50), we included sleep as a possible confounder. Participants wore a wrist actigraph (Micro Mini-Motionlogger, Ambulatory Monitoring, Ardsley, NY) for three days. An actigraph detects arm movement with an accelerometer as a useful proxy for sleep/wake cycles. We stored 60-s epochs, and analyzed using Action 4 (v1.13) and ACT Millennium (beta v3.8.8.9) software (Ambulatory Monitoring, NY). We calculated sleep efficiency as the ratio of time-asleep/time-in-bed averaged over three days, times 100%.
Data Analysis
These data analyses proceeded in the logical steps necessary to test our single hypothesis, examine possible confounders or proxies, and to explore the meaning of our results. In the primary analysis, we used the Cox Proportional Hazards Model to test the effects of resting HF-HRV (as a continuous variable) at baseline on survival (Median 7.99 years). We had no hypothesis that included additional variables in this equation. To provide an indication of the strength of the effect, we show Kaplan-Meier survival curves with a median split on HF-HRV. Our analyses were not meant to explore all variables that might predict survival; rather, to test a single hypothesis, then to see whether variables that both correlated with HF-HRV and significantly predicted survival (therefore possible proxies by the MacArthur definition(51, 52)), could threaten our finding.
Sample descriptive data are in Table 1 with two-tailed 5% significance levels. We explored whether other baseline variables, demographic, medical, depression, stress, fatigue, physical activity, sleep efficiency, BMI, and HR, were possible proxies for another prognostic variable. We explored the associations between HF-HRV and each possible confounder using Spearman correlations, and Cox models to evaluate confounder associations with survival. We explored the joint impacts of HF-HRV and possible proxy variables one at a time (with independent variables HF-HRV, each confounder, and the interaction of HF-HRV and the confounder).
Table 1.
Baseline psychosocial, demographic, and medical status variables for metastatic and recurrent breast cancer patients with (n=87) and without (n=16) HF-HRV data.
| HF-HRV Data | No HF-HRV Data | |||||||
|---|---|---|---|---|---|---|---|---|
| Measure Psychosocial |
N | Mean or % |
SD | N | Mean or % |
SD | P | Effect Size |
| Beck Depression Inventory | 87 | 11.2 | 7.8 | 16 | 12.6 | 7.0 | 0.50 | 0.18 |
| Depressed? (% Yes) | 44/87 | 50.5 | 7/12 | 58.3 | 0.61 | −0.05 | ||
| Perceived Stress Scale | 87 | 16.7 | 7.4 | 16 | 18.2 | 6.2 | 0.45 | 0.21 |
| Previous Sleep Problems? (% Yes) | 34/87 | 39.1 | 6/16 | 37.5 | 0.91 | 0.01 | ||
| Previous Psychiatric Problems? (% Yes) | 16/87 | 18.4 | 4/16 | 25.0 | 0.51 | −0.06 | ||
| Mean Sleep Efficiency | 83 | 0.85 | 0.10 | 14 | 0.83 | 0.14 | 0.47 | 0.21 |
| SF-36 Vitality Scale | 83 | 46.6 | 23.0 | 14 | 47.5 | 22.5 | 0.89 | 0.04 |
| Body Mass Index (kg/m2) | 87 | 25.0 | 4.3 | 16 | 27.4 | 6.0 | 0.057 | 0.52 |
| METs/day | 87 | 39.6 | 6.6 | 11 | 42.6 | 9.8 | 0.34 | 0.43 |
| Demographic | ||||||||
| Age at Baseline (years) | 87 | 54.2 | 9.92 | 16 | 54.88 | 8.06 | 0.81 | 0.07 |
| Ethnicity (% Caucasian) | 67/87 | 77.0 | 14/16 | 87.5 | 0.51 | −0.09 | ||
| Education | 0.79 | −0.03 | ||||||
| High School Diploma or Below | 2 | 2.3 | 1 | 6.3 | ||||
| Trade School or Some College | 32 | 36.8 | 4 | 25.0 | ||||
| Bachelor’s Degree | 18 | 20.7 | 3 | 18.8 | ||||
| Some Graduate School or Master’s | 31 | 35.6 | 8 | 50.0 | ||||
| Ph.D., M.D. or J.D. | 4 | 4.6 | 0 | 0.0 | ||||
| Income | 0.94 | 0.003 | ||||||
| <$20,000 | 4 | 5.2 | 2 | 12.5 | ||||
| $20,000–$39,999 | 14 | 18.2 | 4 | 25.0 | ||||
| $40,000–$59,999 | 12 | 15.6 | 0 | 0 | ||||
| $60,000–$79,999 | 14 | 18.2 | 2 | 12.5 | ||||
| $80,000–$99,999 | 10 | 13.0 | 2 | 12.5 | ||||
| $100,000 or more | 23 | 29.9 | 6 | 37.5 | ||||
| Medical Status | ||||||||
| Disease-Free Interval (months) | 87 | 53.8 | 63.26 | 10 | 108.90 | 89.83 | 0.014* | 0.83 |
| Estrogen Receptor Status (% negative) | 26/86 | 30.2 | 1/10 | 10.0 | 0.27 | 0.14 | ||
| Site of Metastasis/Recurrence | 0.45 | −0.06 | ||||||
| Chestwall | 32 | 36.8 | 2 | 20.0 | ||||
| Bone | 29 | 23.0 | 3 | 30.0 | ||||
| Viscera | 35 | 40.2 | 5 | 50.0 | ||||
| Treatment (% Yes) | ||||||||
| Surgery | 82 | 94.3 | 9 | 90.0 | 0.49 | 0.05 | ||
| Reconstruction | 24 | 27.6 | 2 | 20.0 | 0.99 | 0.05 | ||
| Chemotherapy | 76 | 87.4 | 8 | 80.0 | 0.62 | 0.07 | ||
| Radiation | 72 | 82.8 | 6 | 66.7 | 0.36 | 0.12 | ||
| Hormone Therapy | 57 | 65.5 | 7 | 70.0 | 0.99 | −0.03 | ||
Note. pvalues reported for Students t-test, χ2, Cochran-Armitage Trend Test, or Fisher’s Exact Tests. Effect Size=Cohen’s d, Phi Coefficient, or Kendalls’ Tau-c.
p< .05
Results
At study entry participants were on average age 54.2 (SD=9.92), 77.0% Caucasian, with 39.1% below Bachelor’s degree (Table 1 for demographic, medical, and study variables). Their metastases or recurrence were to chestwall (23.0%), bone (36.8%), and viscera (40.2%). All recurrent diagnoses had cancer spread to either chestwall or bone. Most had surgery (94.3%) with many having reconstructive surgery (27.6%). Most women had chemotherapy (87.4%) and radiation (82.8%), and many used hormone therapy (40.2%). Those with HF-HRV data (n=87) did not differ significantly from those without (n=16) except on disease-free interval (p=0.014, d=0.83), those with HF-HRV having shorter intervals.
Association between Baseline Resting HF-HRV and Survival
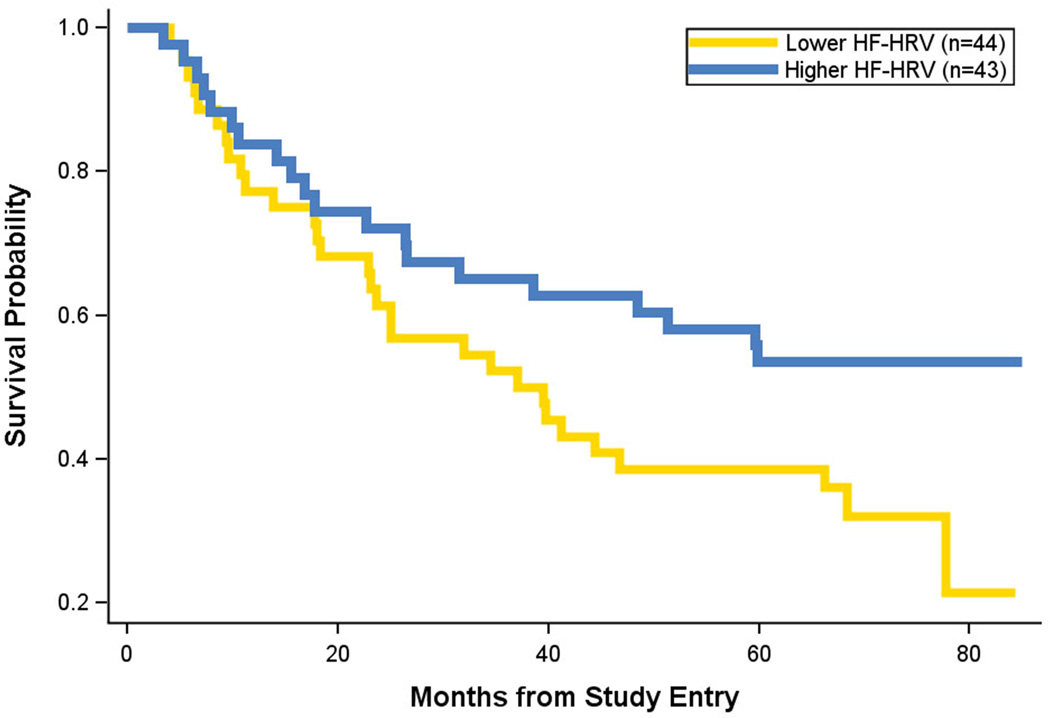
A total of 50 patients died during a median follow-up of 7.99 years. In the primary analysis, (a priori hypothesis) higher baseline resting HF-HRV (mean=4.17, SD=1.33, Median=4.15, Range=0.51–7.28) significantly predicted longer survival, B= −0.294, Wald=7.51, hazard ratio=0.75 (95% CI=0.60 to 0.92; p=.006; Figure 1). At 37.09 months (median survival from the Kaplan-Meier), 50% of the lower HF-HRV group had died, but for women with higher HF-HRV only 34.88% had died.
Figure 1.
Kaplan-Meier survival curve for lower high-frequency heart-rate variability (HF-HRV) (gold line) versus higher HF-HRV (blue line) from resting baseline data. Censored data is removed from the figure. Breast cancer or cancer was the primary or secondary cause of death for 100% of the patients.
Effect of Baseline Demographic/Medical Potential Proxy Variables on HF-HRV
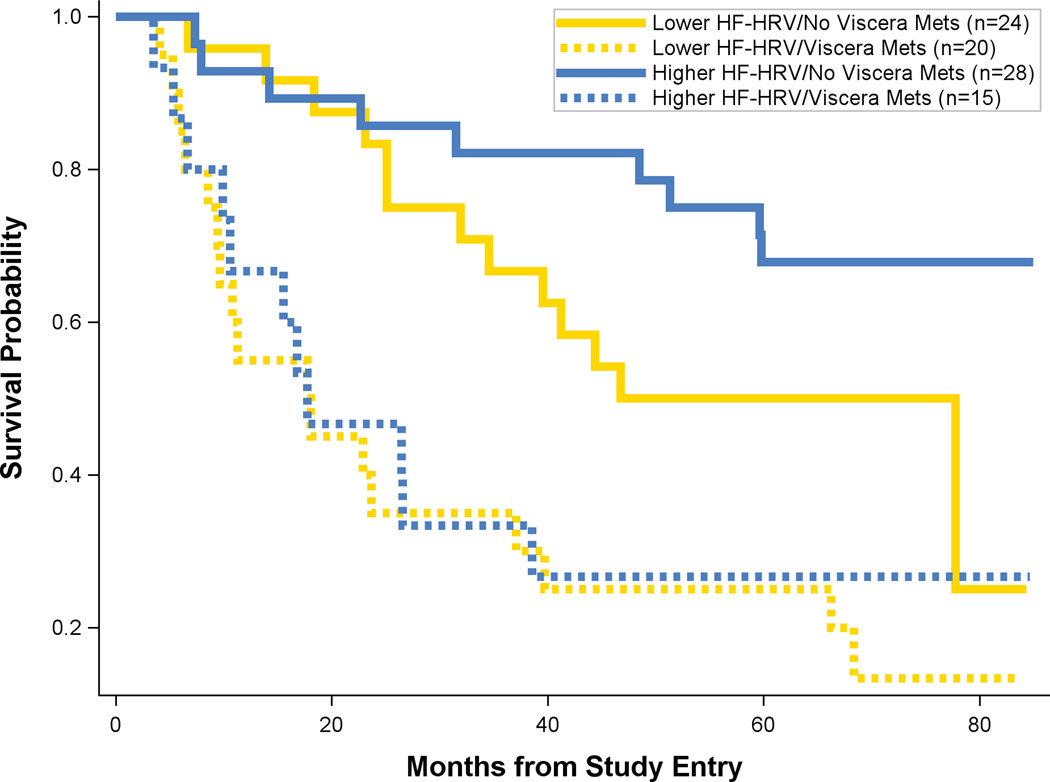
Of all the possible confounders (proxies) measured, the only variables both significantly correlated with HF-HRV and predictive of survival were sleep efficiency(50), visceral metastases, reconstructive surgery, and higher HR (mean=81.22, SD=12.01, Median=81.01, Range=53.39–107.51), to which HF-HRV could possibly be a proxy (Table 2 for correlations). No other variables, including disease stage, met the proxy criteria. Therefore, we re-ran the Cox Proportional Hazards equation with HF-HRV, on possible proxies (one at a time), and their interaction with HF-HRV to test whether the finding of increased survival for HF-HRV would remain. For disease stage, the association of HF-HRV with survival was maintained in analysis that focused only on women with metastatic disease and excluded the 17 women with recurrent disease (B= −0.276, Wald=6.243, hazard ratio=0.756; p=.012). When we considered sleep efficiency, or reconstructive surgery, HF-HRV remained significant. However, when we considered visceral metastasis, we found that HF-HRV may be a proxy (i.e., only the effect of visceral metastasis is significant, with HF-HRV reduced p=.065). When we explored this finding (Figure 2), we found that HF-HRV had little to no effect predicting survival in women with visceral metastases; however, it had a pronounced effect in the combined chestwall-or-bone metastasis group. When we considered HR, neither it nor HF-HRV was statistically significant, probably a consequence of collinearity, which results in an inability to separate the two effects on survival.
Table 2.
Spearman correlations among possible confounders and HF-HRV (n=87).
| HF-HRV | HR | Reconstructive Surgery |
Visceral Metastases |
Sleep Efficiency |
|
|---|---|---|---|---|---|
| HF-HRV | --- | ||||
| HR | −0.43** | --- | |||
| Reconstructive Surgery | 0.23* | −0.16 | --- | ||
| Visceral Metastases | −0.26* | 0.22* | −0.15 | --- | |
| Sleep Efficiency | 0.30** | −0.24* | 0.17 | −0.19 | --- |
p< .05,
p< .01
Figure 2.
Kaplan-Meier survival curves for two factors, high-frequency heart-rate variability (HF-HRV) and visceral metastases versus a combined group of bone and chestwall metastases. Figure shows lower HF-HRV with no visceral metastases (solid gold line) versus higher HF-HRV (solid blue line) with no visceral metastasis. Figure also shows lower HF-HRV with visceral metastasis (dashed gold line) versus higher HF-HRV with visceral metastasis (dashed blue line). Censored data is removed from the figure.
Post-Hoc Analyses
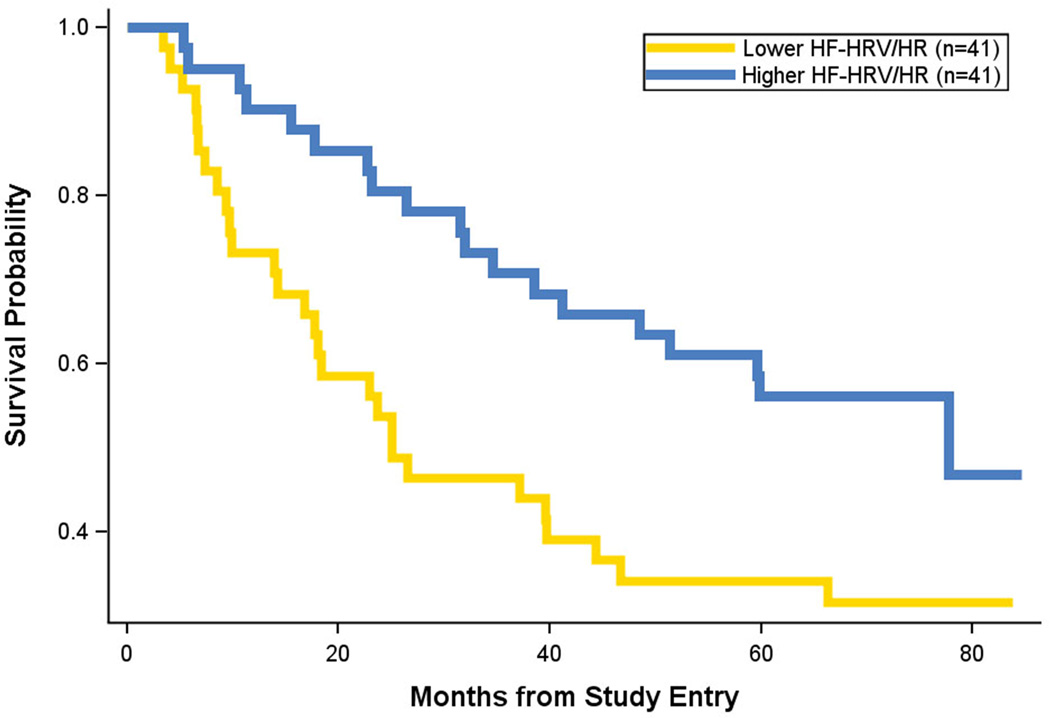
In order to disentangle HF-HRV and HR effects on survival, we conducted a principal components analysis assessing their overlap using Varimax rotation. Both variables loaded on a single factor accounting for 72.9% of the variance (HR correlated negatively with HF-HRV). Using factor scores to create a combined HF-HRV/HR variable (equivalent to standardizing each and taking the mean), we again conducted the Cox Regression on survival. Using the factor scores improved the prediction, B= −0.447, Wald=9.67, hazard ratio=0.64 (95% CI=0.48 to 0.85; p=.002: Figure 3). Re-examining whether HF-HRV (now HF-HRV/HR) was proxy to visceral metastases we now found that both HF-HRV/HR (B= −0.338, Wald=4.94, hazard ratio=0.71, p=.026) and visceral metastases (B=0.97, Wald=9.78, hazard ratio=2.63, p=.002) significantly predicted survival with no significant interaction.
Figure 3.
Kaplan-Meier survival curve for a combined measure of high-frequency heart-rate variability and heart rate (HF-HRV/HR), equivalent to standardizing each measure and taking the mean, where the two measures correlate negatively. Lower HF-HRV/HR (gold line) versus higher HF-HRV/HR (blue line) from resting baseline data. Censored data is removed from the figure
Discussion
Based on a broad literature linking higher vagal activity to longer survival(11, 16–19, 53–58), and emerging connections between vagal modulation of immune function and cancer(2, 4, 5, 11, 13, 59–64), we tested whether resting high-frequency heart-rate variability (HF-HRV) predicted survival in women with metastatic or recurrent breast cancer. As expected, we found that higher resting HF-HRV significantly predicted longer overall survival, establishing HF-HRV as a long-term predictor of cancer survival for the first time. Our analyses point to the theoretical and clinical importance of low HF-HRV as a risk factor for shorter survival in women with a diagnosis of MRBC.
Higher HF-HRV predicts longer survival across many medical conditions(11, 16–19,53–58, 65, 66). It may be a marker of the “inflammatory reflex,”(7)--the vagus nerve informing the brain about tumors and modulating them through feedback to neuroendocrine and immune systems(2, 4, 5, 11, 13, 59–64). Vagal activity also contributes to self-regulation of emotion and social activity(67–71), and is linked to treatment adherence in breast cancer(22). Thus, our finding may be mediated by behavioral factors such as emotional expression, social engagement and support, depression, and adherence to treatment which contribute both to higher vagal activity and to longer cancer survival(72–77).
Our findings extend previous cancer research suggesting HF-HRV-survival associations. We increase prediction time, and improve measurement using established spectral analysis. For instance, in a small-scale study of hospice patients, higher HF-HRV predicted greater 3-month survival(23), indicating (like many trauma studies) that it strongly predicts short-term survival. In prostate cancer, a small 10-s) measurement window predicted a one-year survival outcome(26). Likewise, in a study of men, higher HF-HRV predicted all-cause and cancer mortality, however measurement was substandard (a 15–30 sec manually scored ECG)(27).
We explored variables in our analyses with a potential for being involved in the relationship of HF-HRV and survival as proxies. Degree of visceral metastases appears to modulate the strength of HF-HRV in predicting survival, and should be considered in future studies. The inflammatory reflex can detect and dampen inflammation known to increase tumor growth(62, 78). But once there is significant growth in visceral organs known to facilitate this vagus nerve circuit(62), the inflammatory reflex may have limited impact.
We also found that lower HR predicted longer survival (< median 81 bpm). HR may reflect general arousal due to emotional factors such as stress(79), anxiety(80), or anger(81), and is reduced in physically fit individuals(82). Increases in HR can be the result of decreased vagal activity, increased sympathetic activity, or a combination of these partially independent autonomic pathways(83). Our results indicate relative equivalence of HF-HRV (a vagal measure) and HR (a combined sympathovagal measure) for predicting MRBC mortality, and superior prediction when combined. Thus, the sympathoadrenal pathway likely contributes to reducing survival in the studied cancer patients. Several studies demonstrated relationships between sympathetic activity, the immune system(61, 82, 84), and metastases(85).
Evidence suggests that high HR levels (>100 bpm), may predict shorter survival in cancer and cardiovascular disease(86). Several large-scale studies found that higher HR predicted shorter cancer survival among men(87–90), and also higher HR in combination with lower exercise predicted shorter survival(90). Other studies found no relationship between higher HR and cancer mortality(91–93). Nevertheless, we suggest that a combined measurement of sympathetic and vagal activity may benefit future survival studies.
Evidence in rodents indicates that sympatho-adrenal-medullary system activity, specifically norepinephrine secretion, is associated with vascular endothelial growth factor and blood supply stimulation, and tumor growth(94). This effect was reversed with beta-adrenergic blocker administration. Human and animal models confirm some of those pathways(61, 62,85, 95–100), and several clinical studies have demonstrated that a standard beta-adrenergic blocker, propranalol, is associated with longer breast cancer survival(101–103). To this literature we add evidence that resting HF-HRV/HR, representing the combined influence of increased vagal and decreased sympathetic activity, predicts survival in a clinical sample. This combined measure is easy to assess, potentially giving health-care professionals incremental information on which to base treatment plans.
Limitations
The sample size for this secondary analysis is limited to women with MRBC volunteering to undergo a social-stress test, and was smaller than the larger study powered to test another hypothesis. Replication is necessary for full confidence in this result. Although only disease-free interval differed between the two samples, there may be a selection bias toward less healthy individuals. Our vagal measure indexes efferent vagal activity to the cardiac muscle, and other organs may be differentially innervated by the vagal system depending on local (metabolic) requirements. Also, afferent activity, hypothesized as important to the inflammatory reflex informing the CNS about peripheral inflammation(2), may differ from efferent activity. Despite these limitations, the relationship of HF-HRV with survival indicates that efferent cardiac vagal activity may represent overall afferent and efferent information transfer between the vagal and immune systems and may provide early clinical prognoses in cancer patients.
In sum, our results provide a translational link between basic research on the immunomodulatory function of the vagal system and a clinical population that may be particularly dependent on CNS signalling(85) and responsive to interventions that improve immune function(104, 105). The power of HF-HRV and HF-HRV/HR to predict survival suggests that the autonomic nervous system could be an important target for pharmacological(29), surgical and non-invasive vagus stimulation (106, 107), or behavioral interventions such as HRV and HR biofeedback(30–32), and exercise or insomnia programs(33–36). HF-HRV’s predictive power also recommends it as a clinical diagnostic aid in secondary and tertiary prevention measures, and some already suggest that its use would be a prognostic measurement improvement in hospice settings(24).
Acknowledgements
Portions of this paper were presented at the Annual Meeting of the American Psychosomatic Society, Portland, OR. March 10–12, 2010; Tom Baker Cancer Centre Grand Rounds, March 9, 2011, Calgary, AB, Canada; and the Canadian Association of Psychosocial Oncology, April 24–46, 2013, Ottawa, ON, Canada. We wish to thank Bita Nouriani, research coordinator, for recruiting participants and Ansgar Conrad, for conducting most of the TSST sessions.
Sources of Funding: This study was funded by NIA/NCI Program Project AG18784 to David Spiegel, P.I., and in part by grant 5 M01 RR000070 from the National Center for Research Resources, National Institutes of Health to the GCRC Stanford. The funders had no role in the manuscript. Salary funding for the first author from The Alberta Cancer Foundation and The Enbridge Chair for Psychosocial Oncology Research held by Linda E. Carlson.
Abbreviations
- HF-HRV
High Frequency Heart-Rate Variability
- HRV
Heart-Rate Variability
- HR
Heart Rate
- MRBC
Metastatic and Recurrent Breast Cancer
- TSST
Trier Social Stress Test
- SDNN
Standard deviations of normal-to-normal R-R intervals
- HIPAA
Health Insurance Portability and Accountability Act
- ECG
Electrocardiogram
- METs
Metabolic units
- BDI
Beck Depression Inventory
- SCID
Structured Clinical Interview for DSM Disorders
- MDD
Major Depressive Disorder
- PSS
Perceived Stress Scale
- MOS-SF 36
Medical Outcomes Study 36-Item Short Form Health Survey
- CIs
Confidence Intervals
- BMI
Body Mass Index
- Bpm
beats per minute
- HF-HRV/HR
Combined HF-HRV and HR variable
- CNS
Central Nervous System
Footnotes
Conflicts of Interest: There are no conflicts of interest for any author.
References
- 1.Giese-Davis J, Wilhelm FH, Conrad A, Abercrombie HC, Sephton S, Yutsis M, Neri E, Taylor CB, Kraemer HC, Spiegel D. Depression and stress reactivity in metastatic breast cancer. Psychosom Med. 2006;68:675–683. doi: 10.1097/01.psy.0000238216.88515.e5. [DOI] [PubMed] [Google Scholar]
- 2.Gidron Y, Perry H, Glennie M. Does the vagus nerve inform the brain about preclinical tumours and modulate them? Lancet Oncol. 2005;6:245–248. doi: 10.1016/S1470-2045(05)70096-6. [DOI] [PubMed] [Google Scholar]
- 3.Gidron Y, Ronson A. Psychosocial factors, biological mediators, and cancer prognosis: a new look at an old story. Curr Opin Oncol. 2008;20:386–392. doi: 10.1097/CCO.0b013e3282fbcd0d. [DOI] [PubMed] [Google Scholar]
- 4.Mravec B, Gidron Y, Kukanova B, Bizik J, Kiss A, Hulin I. Neural-endocrine-immune complex in the central modulation of tumorigenesis: facts, assumptions, and hypotheses. J Neuroimmunol. 2006;180:104–116. doi: 10.1016/j.jneuroim.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Golan H, Kennedy JA, Frenkel A, Parmet Y, Feintuch A, Levi O, Gidron Y. Brain mapping of patients with lung cancer and controls: inquiry into tumor-to-brain communication. J Nucl Med. 2009;50:1072–1075. doi: 10.2967/jnumed.108.061085. [DOI] [PubMed] [Google Scholar]
- 6.Olofsson P, Rosas Ballina M, Levine Y, Tracey K. Rethinking inflammation: neural circuits in the regulation of immunity. Immunol Rev. 2012;248:188–204. doi: 10.1111/j.1600-065X.2012.01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tracey K. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 8.Tonhajzerova I, Mokra D, Visnovcova Z. Vagal function indexed by respiratory sinus arrhythmia and cholinergic anti-inflammatory pathway. Respiratory Physiology and Neurobiology. 2013;187:78–81. doi: 10.1016/j.resp.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Mravec B, Gidron Y, Hulin I. Neurobiology of cancer: Interactions between nervous, endocrine and immune systems as a base for monitoring and modulating the tumorigenesis by the brain. Semin Cancer Biol. 2008;18:150–163. doi: 10.1016/j.semcancer.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Mravec B, Ondicova K, Valaskova Z, Gidron Y, Hulin I. Neurobiological principles in the etiopathogenesis of disease: when diseases have a head. Med Sci Monit. 2009;15:RA6–RA16. [PubMed] [Google Scholar]
- 11.De Couck M, Mravec B, Gidron Y. You may need the vagus nerve to understand pathophysiology and to treat diseases. Clin Sci. 2012;122:323–328. doi: 10.1042/CS20110299. [DOI] [PubMed] [Google Scholar]
- 12.Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med. 2011;269:45–53. doi: 10.1111/j.1365-2796.2010.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosas-Ballina M, Tracey K. The neurology of the immune system: neural reflexes regulate immunity. Neuron. 2009;64:28–32. doi: 10.1016/j.neuron.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 15.Thayer J, Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci. 2006;1088:361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- 16.Buccelletti E, Gilardi E, Scaini E, Galiuto L, Persiani R, Biondi A, Basile F, Silveri NG. Heart rate variability and myocardial infarction: systematic literature review and metanalysis. Eur Rev for Med Pharmacol Sci. 2009;13:299–307. [PubMed] [Google Scholar]
- 17.Karp E, Shiyovich A, Zahger D, Gilutz H, Grosbard A, Katz A. Ultra-short-term heart rate variability for early risk stratification following acute ST-elevation myocardial infarction. Cardiology. 2009;114:275–283. doi: 10.1159/000235568. [DOI] [PubMed] [Google Scholar]
- 18.Carney R, Blumenthal J, Stein P, Watkins L, Catellier D, Berkman L, Czajkowski S, O'Connor C, Stone P, Freedland K. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104:2024–2028. doi: 10.1161/hc4201.097834. [DOI] [PubMed] [Google Scholar]
- 19.Riordan WP, Norris PR, Jenkins JM, Morris JA. Early loss of heart rate complexity predicts mortality regardless of mechanism, anatomic location, or severity of injury in 2178 trauma patients. J of Surg Res. 2009;181:106–113. doi: 10.1016/j.jss.2009.03.086. [DOI] [PubMed] [Google Scholar]
- 20.Kogan A, Allen JJB, Weihs K. Cardiac vagal control as a prospective predictor of anxiety in women diagnosed with breast cancer. Biol Psychol. 2012;90:105–111. doi: 10.1016/j.biopsycho.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 21.Fagundes C, Murray D, Hwang B, Gouin J-P, Thayer J, Sollers J, Shapiro C, Malarkey W, Kiecolt Glaser J. Sympathetic and parasympathetic activity in cancer-related fatigue: more evidence for a physiological substrate in cancer survivors. Psychoneuroendocrinology. 2011;36:1137–1147. doi: 10.1016/j.psyneuen.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karvinen K, Murray N, Arastu H, Allison R. Stress reactivity, health behaviors, and compliance to medical care in breast cancer survivors. Oncol Nurs Forum. 2013;40:149–156. doi: 10.1188/13.ONF.149-156. [DOI] [PubMed] [Google Scholar]
- 23.Chiang J-K, Koo M, Kuo TBJ, Fu C-H. Association between cardiovascular autonomic functions and time to death in patients with terminal hepatocellular carcinoma. J Pain Symptom Manage. 2010;39:673–679. doi: 10.1016/j.jpainsymman.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Kim D, Kim J, Choi Y, Kim S, Lee J, Kim Y. Heart rate variability and length of survival in hospice cancer patients. J Korean Med Sci. 2010;25:1140–1145. doi: 10.3346/jkms.2010.25.8.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mouton C, Ronson A, Razavi D, Delhaye F, Kupper N, Paesmans M, Moreau M, Nogaret J-M, Hendlisz A, Gidron Y. The relationship between heart rate variability and time-course of carcinoembryonic antigen in colorectal cancer. Auton Neurosci. 2012;166:96–99. doi: 10.1016/j.autneu.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 26.De Couck M, van Brummelen D, Schallier D, De-GrÃve J, Gidron Y. The relationship between vagal nerve activity and clinical outcomes in prostate and non-small cell lung cancer patients. Oncology Reports. 2013;30:2435–2441. doi: 10.3892/or.2013.2725. [DOI] [PubMed] [Google Scholar]
- 27.Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men. Am J Epidemiol. 1997;145:899–908. doi: 10.1093/oxfordjournals.aje.a009049. [DOI] [PubMed] [Google Scholar]
- 28.Palesh O, Aldridge-Gerry A, Zeitzer JM, Koopman C, Neri E, Giese-Davis J, Jo B, Kraemer HC, Nouriani B, Spiegel D. Actigraphy Measured Sleep Disruption as a Predictor of Survival Among Women with Advanced Breast Cancer. Sleep. doi: 10.5665/sleep.3642. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernik TR, Friedman SG, Ochani M, DiRaimo R, Ulloa L, Yang H, Sudan S, Czura CJ, Ivanova SM, Tracey KJ. Pharmacological stimulation of the cholinergic antiinflammatory pathway. The Journal of experimental medicine. 2002;195:781–788. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehrer P, Karavidas M, Lu S-E, Coyle S, Oikawa L, Macor M, Calvano S, Lowry S. Voluntarily produced increases in heart rate variability modulate autonomic effects of endotoxin induced systemic inflammation: an exploratory study. Appl Psychophysiol Biofeedback. 2010;35:303–315. doi: 10.1007/s10484-010-9139-5. [DOI] [PubMed] [Google Scholar]
- 31.Karavidas M, Lehrer P, Vaschillo E, Vaschillo B, Marin H, Buyske S, Malinovsky I, Radvanski D, Hassett A. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Appl Psychophysiol Biofeedback. 2007;32:19–30. doi: 10.1007/s10484-006-9029-z. [DOI] [PubMed] [Google Scholar]
- 32.Lehrer PM, Vaschillo E, Vaschillo B, Lu SE, Scardella A, Siddique M, Habib RH. Biofeedback treatment for asthma. Chest. 2004;126:352–361. doi: 10.1378/chest.126.2.352. [DOI] [PubMed] [Google Scholar]
- 33.Niederer D, Vogt L, Thiel C, Schmidt K, Bernhorster M, Lungwitz A, Jager E, Banzer W. Exercise effects on HRV in cancer patients. Int J Sports Med. 2013;34:68–73. doi: 10.1055/s-0032-1314816. [DOI] [PubMed] [Google Scholar]
- 34.Iellamo F, Manzi V, Caminiti G, Sposato B, Massaro M, Cerrito A, Rosano G, Volterrani M. Dose-response relationship of baroreflex sensitivity and heart rate variability to individually-tailored exercise training in patients with heart failure. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.10.082. [DOI] [PubMed] [Google Scholar]
- 35.Earnest C, Blair S, Church T. Heart rate variability and exercise in aging women. J Women's Health. 2012;21:334–339. doi: 10.1089/jwh.2011.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palesh O, Zeitzer JM, Conrad A, Giese-Davis J, Mustian KM, Popek V, Nga K, Spiegel D. Vagal regulation, cortisol, and sleep disruption in women with metastatic breast cancer. J Clin Sleep Med. 2008;4:441–449. [PMC free article] [PubMed] [Google Scholar]
- 37.Spiegel D, Giese-Davis J, Taylor CB, Kraemer H. Stress sensitivity in metastatic breast cancer: analysis of hypothalamic-pituitary-adrenal axis function. Psychoneuroendocrinology. 2006;31:1231–1244. doi: 10.1016/j.psyneuen.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test'--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 39.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 40.Taylor CB, Conrad A, Wilhelm FW, Neri E, DeLorenzo A, Kramer MA, Giese-Davis J, Roth W, Oka R, Cooke JP, Kraemer H, Spiegel D. Psychophysiological and cortisol responses to psychological stress in depressed and non-depressed older men and women with elevated CVD risk. Psychosom Med. 2006;68:538–546. doi: 10.1097/01.psy.0000222372.16274.92. [DOI] [PubMed] [Google Scholar]
- 41.Wilhelm FH, Grossman P, Roth WT. Analysis of cardiovascular regulation. Biomed Sci Instrum. 1999;35:135–140. [PubMed] [Google Scholar]
- 42.Welch PD. The use of the fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans Audio and Electroacoust. 1967;15:70–73. [Google Scholar]
- 43.Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol. 2007;74:263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Sidney S, Jacobs DR, Haskell WL, Armstrong MA, Dimicco A, Oberman A, Savage PJ, Slattery ML, Sternfeld B, Van Horn L. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1991;133:1231–1245. doi: 10.1093/oxfordjournals.aje.a115835. [DOI] [PubMed] [Google Scholar]
- 45.Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, Paffenbarger RS. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121:91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 46.Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Research quarterly for exercise and sport. 2000;71:S1–S14. [PubMed] [Google Scholar]
- 47.Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- 48.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 49.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 50.Palesh O, Aldridge Gerry A, Zeitzer J, Koopman C, Neri E, Giese Davis J, Jo B, Kraemer H, Nouriani B, Spiegel D. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep. 2014;37:837–842. doi: 10.5665/sleep.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kraemer H, Kiernan M, Essex M, Kupfer D. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychology. 2008;27:S101–S108. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. American Journal of Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- 53.Chen WL, Tsai TH, Huang CC, Chen JH, Kuo CD. Heart rate variability predicts short-term outcome for successfully resuscitated patients with out-of-hospital cardiac arrest. Resuscitation. 2009;80:1114–1118. doi: 10.1016/j.resuscitation.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 54.Lanza GA, Cianflone D, Rebuzzi AG, Angeloni G, Sestito A, Ciriello G, La Torre G, Crea F, Maseri A. Prognostic value of ventricular arrhythmias and heart rate variability in patients with unstable angina. Heart. 2006;92:1055–1063. doi: 10.1136/hrt.2005.070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Norris PR, Canter JA, Jenkins JM, Moore JH, Williams AE, Morris JA. Personalized medicine: genetic variation and loss of physiologic complexity are associated with mortality in 644 trauma patients. Ann Surg. 2009;250:524–530. doi: 10.1097/SLA.0b013e3181b8fb1f. [DOI] [PubMed] [Google Scholar]
- 56.King D, Ogilvie M, Pereira BMT, Chang Y, Manning R, Conner J, Schulman C, McKenney M, Proctor K. Heart rate variability as a triage tool in patients with trauma during prehospital helicopter transport. J Trauma. 2009;67:436–440. doi: 10.1097/TA.0b013e3181ad67de. [DOI] [PubMed] [Google Scholar]
- 57.Leino J, Virtanen M, Kahanen M, Nikus K, Lehtimaki T, Kaabi T, Lehtinen R, Turjanmaa V, Viik J, Nieminen T. Exercise-test-related heart rate variability and mortality: the Finnish Cardiovascular Study. Int J of Cardiol. 2010;144:154–155. doi: 10.1016/j.ijcard.2008.12.123. [DOI] [PubMed] [Google Scholar]
- 58.Tsuji H, Venditti FJ, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation. 1994;90:878–883. doi: 10.1161/01.cir.90.2.878. [DOI] [PubMed] [Google Scholar]
- 59.Gidron Y, Kupper N, Kwaijtaal M, Winter J, Denollet J. Vagus-brain communication in atherosclerosis-related inflammation: a neuroimmunomodulation perspective of CAD. Atherosclerosis. 2007;195:e1–e9. doi: 10.1016/j.atherosclerosis.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 60.Wong CH, Jenne CN, Lee WY, Leger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–105. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- 61.Irwin M, Cole S. Reciprocal regulation of the neural and innate immune systems. Nature Rev Immunol. 2011;11:625–632. doi: 10.1038/nri3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98–101. doi: 10.1126/science.1209985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009;265:663–679. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zappala G, McDonald PG, Cole SW. Tumor dormancy and the neuroendocrine system: an undisclosed connection? Cancer Metastasis Rev. 2013;32:189–200. doi: 10.1007/s10555-012-9400-x. [DOI] [PubMed] [Google Scholar]
- 65.Balogh S, Fitzpatrick DF, Hendricks SE, Paige SR. Increases in heart rate variability with successful treatment in patients with major depressive disorder. Psychopharmacol Bull. 1993;29:201–206. [PubMed] [Google Scholar]
- 66.Dalack GW, Roose SP. Perspectives on the relationship between cardiovascular disease and affective disorder. J Clin Psychiatry. 1990;51:S4–S9. [PubMed] [Google Scholar]
- 67.Porges SW. Emotion: an evolutionary by-product of the neural regulation of the autonomic nervous system. Ann N Y Acad Sci. 1997;807:62–77. doi: 10.1111/j.1749-6632.1997.tb51913.x. [DOI] [PubMed] [Google Scholar]
- 68.Thayer JF, Ahs F, Fredrikson M, Sollers JJ, 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 69.Lane R, McRae K, Reiman E, Chen K, Ahern G, Thayer J. Neural correlates of heart rate variability during emotion. Neuroimage. 2009;44:213–222. doi: 10.1016/j.neuroimage.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 70.Hopp H, Shallcross A, Ford B, Troy A, Wilhelm F, Mauss I. High cardiac vagal control protects against future depressive symptoms under conditions of high social support. Biol Psychol. 2013;93:143–149. doi: 10.1016/j.biopsycho.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolff B, Wadsworth M, Wilhelm FH, Mauss IB. Children's vagal regulatory capacity predicts attenuated sympathetic stress reactivity in a socially supportive context: evidence for a protective effect of the vagal system. Dev Psychopathol. 2012;24:677–689. doi: 10.1017/S0954579412000247. [DOI] [PubMed] [Google Scholar]
- 72.Weihs KL, Enright TM, Simmens SJ. Close relationships and emotional processing predict decreased mortality in women with breast cancer: preliminary evidence. Psychosom Med. 2008;70:117–124. doi: 10.1097/PSY.0b013e31815c25cf. [DOI] [PubMed] [Google Scholar]
- 73.Lutgendorf S, De Geest K, Bender D, Ahmed A, Goodheart M, Dahmoush L, Zimmerman MB, Penedo F, Lucci J, Ganjei Azar P, Thaker P, Mendez L, Lubaroff D, Slavich G, Cole S, Sood A. Social influences on clinical outcomes of patients with ovarian cancer. J Clin Oncol. 2012;30:2885–2890. doi: 10.1200/JCO.2011.39.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. Journal of Clinical Oncology. 2006;24:1105–1111. doi: 10.1200/JCO.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- 75.Giese-Davis J, Spiegel D. Emotional expression and cancer progression. In: Davidson RJ, Scherer KR, Hill Goldsmith H, editors. Handbook of Affective Sciences. Oxford: Oxford University Press; 2003. pp. 1053–1082. [Google Scholar]
- 76.DiMatteo MR, Lepper HS, Groghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Int Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 77.Giese-Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011;29:413–420. doi: 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sarkar C, Chakroborty D, Basu S. Neurotransmitters as regulators of tumor angiogenesis and immunity: the role of catecholamines. Journal of Neuroimmune Pharmacology. 2013;8:7–14. doi: 10.1007/s11481-012-9395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fisher A, Newman M. Heart rate and autonomic response to stress after experimental induction of worry versus relaxation in healthy, high-worry, and generalized anxiety disorder individuals. Biol Psychol. 2013;93:65–74. doi: 10.1016/j.biopsycho.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 80.Pittig A, Arch JJ, Lam CWR, Craske MG. Heart rate and heart rate variability in panic, social anxiety, obsessive–compulsive, and generalized anxiety disorders at baseline and in response to relaxation and hyperventilation. Int J Psychophysiol. 2013;87:19–27. doi: 10.1016/j.ijpsycho.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 81.Fernandez C, Pascual J, Soler J, Elices M, Portella M, Fernandez-Abascal E. Physiological responses induced by emotion-eliciting films. Appl Psychophysiol Biofeedback. 2012;37:73–79. doi: 10.1007/s10484-012-9180-7. [DOI] [PubMed] [Google Scholar]
- 82.Murray DR, Irwin M, Rearden CA, Ziegler M, Motulsky H, Maisel AS. Sympathetic and immune interactions during dynamic exercise. Mediation via a beta 2-adrenergic-dependent mechanism. Circulation. 1992;86:203–213. doi: 10.1161/01.cir.86.1.203. [DOI] [PubMed] [Google Scholar]
- 83.Berntson GG, Cacioppo JT, Quigley KS. Cardiac psychophysiology and autonomic space in humans: empirical perspectives and conceptual implications. Psychol Bull. 1993;114:296–322. doi: 10.1037/0033-2909.114.2.296. [DOI] [PubMed] [Google Scholar]
- 84.Benschop RJ, Nieuwenhuis EE, Tromp EA, Godaert GL, Ballieux RE, van Doornen LJ. Effects of beta-adrenergic blockade on immunologic and cardiovascular changes induced by mental stress. Circulation. 1994;89:762–769. doi: 10.1161/01.cir.89.2.762. [DOI] [PubMed] [Google Scholar]
- 85.Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JMG, Morizono K, Karanikolas BDW, Wu L, Sood AK, Cole SW. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Taylor CB. Depression, heart rate related variables and cardiovascular disease. Int J Psychophysiol. 2010;78:80–88. doi: 10.1016/j.ijpsycho.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 87.Greenland P, Daviglus ML, Dyer AR, Liu K, Huang CF, Goldberger JJ, Stamler J. Resting heart rate is a risk factor for cardiovascular and noncardiovascular mortality: the Chicago Heart Association Detection Project in Industry. Am J Epidemiol. 1999;149:853–862. doi: 10.1093/oxfordjournals.aje.a009901. [DOI] [PubMed] [Google Scholar]
- 88.Jouven X, Escolano S, Celermajer D, Empana JP, Bingham A, Hermine O, Desnos M, Perier MC, Marijon E, Ducimetiere P. Heart rate and risk of cancer death in healthy men. PloS One. 2011;6:e21310. doi: 10.1371/journal.pone.0021310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thomas F, Bean K, Provost JC, Guize L, Benetos A. Combined effects of heart rate and pulse pressure on cardiovascular mortality according to age. J Hypertens. 2001;19:863–869. doi: 10.1097/00004872-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 90.Wannamethee G, Shaper AG, Macfarlane PW. Heart rate, physical activity, and mortality from cancer and other noncardiovascular diseases. Am J Epidemiol. 1993;137:735–748. doi: 10.1093/oxfordjournals.aje.a116734. [DOI] [PubMed] [Google Scholar]
- 91.Nilsson PM, Nilsson JA, Hedblad B, Berglund G. Sleep disturbance in association with elevated pulse rate for prediction of mortality--consequences of mental strain? J Intern Med. 2001;250:521–529. doi: 10.1046/j.1365-2796.2001.00913.x. [DOI] [PubMed] [Google Scholar]
- 92.Kristal-Boneh E, Silber H, Harari G, Froom P. The association of resting heart rate with cardiovascular, cancer and all-cause mortality. Eight year follow-up of 3527 male Israeli employees (the CORDIS Study) Eur Heart J. 2000;21:116–124. doi: 10.1053/euhj.1999.1741. [DOI] [PubMed] [Google Scholar]
- 93.Mensink GB, Hoffmeister H. The relationship between resting heart rate and all-cause, cardiovascular and cancer mortality. Eur Heart J. 1997;18:1404–1410. doi: 10.1093/oxfordjournals.eurheartj.a015465. [DOI] [PubMed] [Google Scholar]
- 94.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, Jennings NB, Armaiz-Pena G, Bankson JA, Ravoori M, Merritt WM, Lin YG, Mangala LS, Kim TJ, Coleman RL, Landen CN, Li Y, Felix E, Sanguino AM, Newman RA, Lloyd M, Gershenson DM, Kundra V, Lopez-Berestein G, Lutgendorf SK, Cole SW, Sood AK. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 95.Cole S. Nervous system regulation of the cancer genome. Brain Behav Immun. 2013;30:S10–S18. doi: 10.1016/j.bbi.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cole SW, Arevalo JMG, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, Sheridan JF, Seeman TE. Computational identification of gene–social environment interaction at the human IL6 locus. Proc Natl Acad Sci U S A. 2010;107:5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cole S. Social regulation of leukocyte homeostasis: the role of glucocorticoid sensitivity. Brain Behav Immun. 2008;22:1049–1055. doi: 10.1016/j.bbi.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thayer JF, Fischer JE. Heart rate variability, overnight urinary norepinephrine and C-reactive protein: evidence for the cholinergic anti-inflammatory pathway in healthy human adults. J Intern Med. 2009;265:439–447. doi: 10.1111/j.1365-2796.2008.02023.x. [DOI] [PubMed] [Google Scholar]
- 99.Sajadieh A, Nielsen OW, Rasmussen V, Hein HO, Abedini S, Hansen JF. Increased heart rate and reduced heart-rate variability are associated with subclinical inflammation in middle-aged and elderly subjects with no apparent heart disease. Eur Heart J. 2004;25:363–370. doi: 10.1016/j.ehj.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 100.Cohen L, Cole S, Sood A, Prinsloo S, Kirschbaum C, Arevalo JMG, Jennings N, Scott S, Vence L, Wei Q, Kentor D, Radvanyi L, Tannir N, Jonasch E, Tamboli P, Pisters L. Depressive symptoms and cortisol rhythmicity predict survival in patients with renal cell carcinoma: role of inflammatory signaling. PloS One. 2012;7:e42324. doi: 10.1371/journal.pone.0042324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Powe DG, Voss MJ, Zanker KS, Habashy HO, Green AR, Ellis IO, Entschladen F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–638. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol. 2011;29:2635–2644. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 103.Pasquier E, Ciccolini J, Carre M, Giacometti S, Fanciullino R, Pouchy C, Montero MP, Serdjebi C, Kavallaris M, Andre N. Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: implication in breast cancer treatment. Oncotarget. 2011;2:797–809. doi: 10.18632/oncotarget.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thornton L, Andersen B, Carson W. Immune, endocrine, and behavioral precursors to breast cancer recurrence: a case-control analysis. Cancer Immunol Immunother. 2008;57:1471–1481. doi: 10.1007/s00262-008-0485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thornton LM, Andersen BL, Schuler TA, Carson WE., III A psychological intervention reduces inflammatory markers by alleviating depressive symptoms: secondary analysis of a randomized controlled trial. Psychosom Med. 2009;71:715–724. doi: 10.1097/PSY.0b013e3181b0545c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singh JP, Kandala J, John Camm A. Non-pharmacological modulation of the autonomic tone to treat heart failure. European Heart Journal. 2014;35:77–85. doi: 10.1093/eurheartj/eht436. [DOI] [PubMed] [Google Scholar]
- 107.Bonaz B, Picq C, Sinniger V, Mayol JF, Clarencon D. Vagus nerve stimulation: from epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterology and motility. 2013;25:208–221. doi: 10.1111/nmo.12076. [DOI] [PubMed] [Google Scholar]