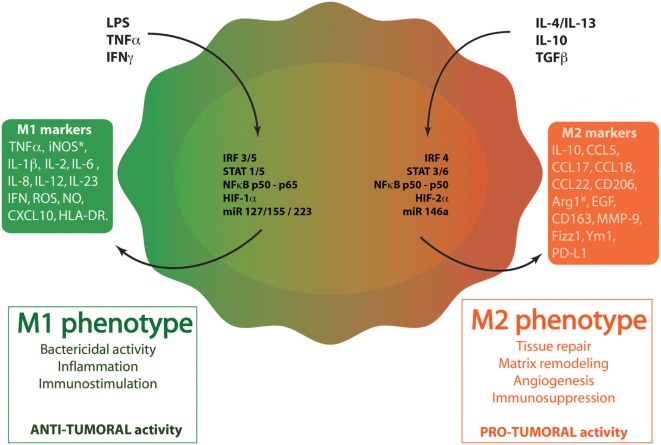
Figure 1.
Macrophage polarization. Through the binding to their respective receptors, M1 stimuli [lipopolysaccharide (LPS), tumor necrosis factor α (TNFα), and interferon γ (IFNγ)] trigger the activation of several transcription factors. These factors include interferon-regulatory factor/signal transducer and activator of transcription (IRF/STAT) family members (IRF3, IRF5, STAT1, and STAT5), the active nuclear factor kappa B (NFκB) heterodimer (p50–p65) and HIF1. miR127, miR 155, and miR223 also regulates M1 polarization. When polarized in M1-like phenotype, macrophages produce specific cytokines (TNFα, IL-1β, IL-2, IL-6, IL-12, IL-23, IFNγ), chemokines (CXCL10) and other molecules [reactive oxygen species (ROS), nitric oxide (NO), inducible nitric oxide synthase (iNOS), human leukocyte antigen-cell surface receptor (HLA-DR)]. M1 phenotype plays key roles in inflammation, immunostimulation and an antibacterial and antitumoral responses. M2 stimuli [IL-4, IL-13, IL-10, and transforming growth factor β (TGFβ)] bind to ILR4α, ILR10, or TGFβR to induce M2-like phenotype in macrophages. These stimuli activate several transcription factors: IRF/STAT family members (IRF4, STAT 3, and STAT6), the inhibitory NFκB homodimer (p50–p50) and HIF2. miR14a also influences M2 polarization. When polarized in M2-like phenotype, macrophages produce specific cytokines (IL-10), chemokines (CCL5, CCL17, CCL18, CCL22), and other proteins (CD163, CD206, Arg1, MMP-9, Fizz-1, Ym-1, and PD-L1). M2 macrophages exert diverse functions, such as tissue repair, matrix remodeling, angiogenesis, immunosuppression, and favor tumor growth.