Figure 3. Ribosome dimerization occurs via interaction of the C‐terminal domain of SaHPF, and via interactions involving h26.
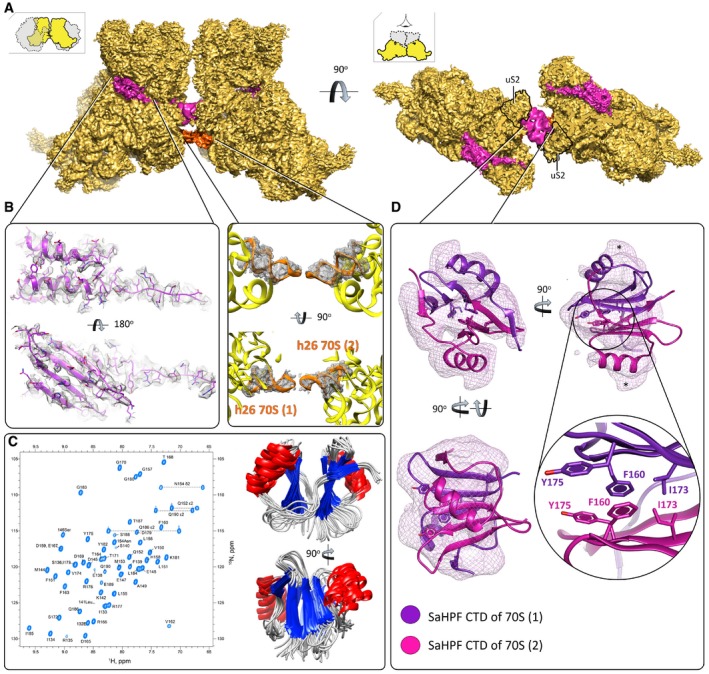
- View of the density maps for unrotated ribosome‐bound SaHPF, emphasizing the dimer interface (magenta, SaHPF; orange, h26; LSU omitted for clarity; orientations as in Fig 2). The density for CTD (interface) derives from the same but Gaussian‐filtered (2.0 SD) high‐resolution map.
- Side chains of amino acids from the N‐terminal domain of SaHPF are visible in the density map (left; unfiltered map). Helix 26 stabilizes dimer formation in the “tight” dimer (center; unfiltered map).
- SaHPF‐CTD forms a dimer in solution. 15N,1H‐HSQC spectrum of the C‐terminal domain of SaHPF showing backbone amide resonances (left). The spectrum was recorded at a proton resonance frequency of 700 MHz at 35°C, in PBS buffer (90% H2O + 10% D2O) at pH 7.6 with 200 mM NH4Cl concentration. Backbone superimposition of the 10 final simulated annealing structures in two orientations (right).
- Dimerization of the C‐terminal domain of SaHPF from two ribosomes within a dimer (map filtered at 2.0 SD using a Gaussian filter). Residues involved in hydrophobic interactions at the interface of the two CTDs are shown as sticks. The region of potential interaction of CTD with bS2 is marked by *.
