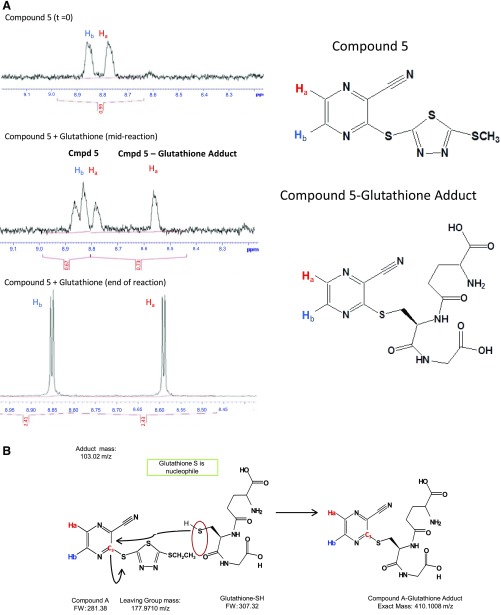
Fig. 6.
NMR analysis of the compound 5–glutathione adduct. (A) NMR spectra (left) of the region corresponding to two hydrogen groups (Ha and Hb) of compound 5 before the reaction (top), midreaction (24 hours; middle), and after completion of reaction (48 hours; bottom) with glutathione, showing a shift in the spectra for Ha after derivitization with GSH. (B) Scheme for reaction mechanism of GSH with compound A. Carbon 3 of compound A (FW 281.38) undergoes nucleophilic attack by the free sulfhydryl group of glutathione-SH (FW 307.32), leading to a compound A–GSH adduct of 410.1008 m/z. FW, Formula weight.