Abstract
Objective
Chronic rhinosinusitis (CRS) is a variable multifactorial disease. It can be divided into forms with nasal polyps (CRSwNP) and without (CRSsNP). Sinus and/or nasal polypectomy surgery are considered if maximal conservative treatment is insufficient. The predictive factors of the need of revision surgery comprise mostly the CRSwNP phenotype and are not fully understood.
Study Design
The aim of this follow‐up study was to evaluate the factors associated with the revision surgery rate in CRS patients with variable extent of disease.
Methods
Data of CRS patients (N = 178) undergoing sinus surgery and/or nasal polypectomy in 2001 to 2010 were used. Patient characteristics and follow‐up data were collected from patient records and questionnaires. Associations were analyzed by Fisher's exact, Mann Whitney U, and the Kaplan‐Meier method with log‐rank test. Unadjusted Cox's proportional hazard models were used for 12 variables and were fitted for the need for revision sinus surgery and/or nasal polypectomy during follow‐up of in average 9 years.
Results
The proportion of CRS patients who had undergone revision in 5 years was 9.6%. After adjustment, the following factors associated significantly with the need for recurrent CRS surgery: allergic rhinitis, corticosteroid treatment, previous surgery of CRS, and recurrent NP.
Conclusion
Increased risk of progressive CRS phenotypes with the need for revision surgery would putatively be recognized by relatively simple clinical questions. Further studies with increased sample size are needed to evaluate whether these predictive factors would be relevant for developing better detection and management of progressive CRS.
Level of Evidence
2b.
Keywords: Antrochoanal polyp, aspirin‐exacerbated respiratory disease, aspirin intolerance, inflammation, nasal polyp, sinusitis, sinus surgery, recurrence, revision surgery
INTRODUCTION
Chronic rhinosinusitis (CRS) is a heterogeneous group of inflammatory diseases of the nose and paranasal sinuses lasting for at least 12 weeks1; it is one of the most common chronic adult health problems in the Western world. A European study estimated the prevalence of chronic rhinosinusitis (CRS) at 11%, although a prevalence of 6% to 7% has been observed in studies with physician‐led diagnosis of CRS.2, 3, 4, 5 Chronic rhinosinusitis has a severe impact on quality of life that is comparable with other chronic diseases such as asthma, chronic obstructive pulmonary disease, and diabetes.1 The economic burden caused by CRS is significant and relates largely to loss of productivity, increased doctor's appointments, and medical expenses. In United States, the CRS‐related healthcare costs are estimated to be $6.9 to $9.9 billion dollars (USD) per year.6
The predisposing factors of CRS include genetic factors, anatomic abnormalities, airborne irritants/allergens/microbes, and host immunity.7 Chronic rhinosinusitis with nasal polyps (CRSwNP) and without (CRSsNP) are considered to be phenotypes of CRS with possibly different etiologies and pathomechanisms.1 However, they may also be interpreted as different degrees of inflammation.1 CRSwNP affects between 1% and 4% of the general population.1 Aspirin‐exacerbated respiratory disease (AERD) has been reported in 8% to 26% of patients with CRSwNP8; it is characterized by severe eosinophilic hyperplastic inflammation of all sinuses and nasal passages, as well as of the lower airways.9
Endoscopic sinus surgery (ESS) has been the most common type of surgery for rhinosinusitis for those with failed maximal medical treatments.1 The variation in using operative management in CRS might reflect a number of factors, including both underutilization and overutilization of surgery, a lack of guidelines, and a lack of evidence in well‐constructed randomized controlled trials (RCTs) .2 The Cochrane collaboration stated in 2006 that ESS offers no additional benefit in relieving symptoms of CRS compared to medical treatment.10 At that time, only six randomized controlled trials were available. Although level 1 evidence is lacking, recent studies evaluating the symptomatic and economic benefits of surgical intervention in CRS favor surgical intervention over ongoing medical therapy.2, 11, 12 Studies also suggest that patients with prolonged and chronic disease might benefit more from surgery than continued medical therapy.6
Endoscopic sinus surgery usually has high initial success rate.1 Published success rates for ESS range from 76% to 98%.13 Common failure factors associated with ESS include inappropriate surgical technique, poor operative field or visualization, and inadequate postoperative care.13 Revision ESS is likely to pose more surgical risks than primary ESS because major complication rates approximate 1%.14, 15 Revision rates range from 3% to 20%, depending mostly on the population and follow‐up time.14, 15 According to a large prospective cohort study, approximately 20% of patients responded unsatisfactorily to surgery and required revision during the 5‐year follow‐up.15 In this study, 20.6% of the patients with polyps had revision surgery in the previous 5 years compared to 15.5% of the patients with CRS alone.15
A few patients have progressive and recurrent forms of CRS, which is challenging for patients and clinicians.16 Previous studies have mostly been performed in CRSwNP phenotype and have shown that the following factors associate with the frequent revisions of CRS: male gender, young adults, smoking, occupational exposure, radiological inflammatory findings in frontal sinus(es), presence of nasal polyps, asthma, and AERD.16, 17, 18, 19, 20, 21, 22 There is a need to evaluate factors associated with the revision rate in several types of CRS. The purpose of this study is to identify the clinical factors that influence the number and time to revision sinus surgery and/or nasal polypectomy in heterogeneous group of CRS patients.
MATERIALS AND METHODS
Patients
This study was performed at the Departments of Otorhinolaryngology, Tampere University Hospital, Päijät‐Häme Central Hospital, Mikkeli Central Hospital, Tampere City Hospital, and Haartman Institute, University of Helsinki, Finland. The study protocol was approved by the Ethical Committee of Pirkanmaa Hospital District and the Administration of the Hospital. Written informed consent was not required for the retrospective study populations (II–III) and for the collection of follow‐up data from patient records of study I population. The study population was composed of different phenotypes of CRS patients who had undergone to variable extent sinus surgery and/or polypectomy in 2001 to 2010 (N = 205). The follow‐up data was available from the hospital's patient records or questionnaires from a total of 178 (87%) CRS patients. The CRS patients were from three preexisting studies.23, 24, 25, 26 The first study data was collected from an initial study, which was a prospective follow‐up study that included two sets of patients who underwent maxillary sinus surgery between 2001 to 2002. Of those, 32 CRS patients (16 previously operated) underwent maxillary sinus surgery ± ethmoidectomy ± nasal polypectomy, and 25 non‐previously operated CRSsNP patients underwent simple uncinectomy‐only on one side and simple uncinectomy with middle meatal antrostomy on the other side.25, 26 Written informed consent from the initial first prospective study patients was obtained. The second study was a retrospective follow‐up study of 63 CRSwNP patients (16 previously operated) who underwent nasal polypectomy ± sinus surgery between 2005 to 2006.23 Eight of these CRSwNP patients had antrochoanal polyps (ACP) and were a part of a random sample of our hospital's nasal polyp cases. The third study was a retrospective follow‐up study of 58 nonoperated CRSsNP patients. Of these, 28 had undergone simple maxillary balloon sinuplasty and 30 simple middle‐meatal antrostomy between 2008 to 2010.24 Diagnosis of CRSsNP, CRSwNP, or ACP was based on the criteria of the European position paper on rhinosinusitis and nasal polyps (EPOS) for symptoms, endoscopic, and sinus computed tomography findings were based on the patient records.1 The exclusion criteria in all cohorts were cystic fibrosis and primary ciliary dyskinesia.
Data Collection
Patient records were abstracted for time until revision surgery and variables that could potentially influence the need for revision surgery: age; gender; asthma diagnosis; AERD; AR; current/recurrent NP; previous sinus surgery and/or nasal polypectomy operation(s); smoking history; occupational exposure; need for regular intranasal corticosteroids; preoperatively administered peroral corticosteroid course; and the initial study operation (only polypectomy, ESS ± polypectomy, only maxillary mini‐invasive ESS e.g. uncinectomy‐only on one side, only maxillary balloon sinuplasty). The number of revision sinus operations and/or revision nasal polypectomies during follow‐up were calculated. Time until the first revision sinus surgery and/or nasal polypectomy was defined a priori as the time between the initial study operation and the first revision sinus surgery and/or nasal polypectomy during follow‐up.
Diagnosis of allergic rhinitis was based on subject‐reported doctor‐diagnosed AR and/or skin prick test positivity and report of typical symptoms following exposure to allergen to which sensitized. Diagnosis of asthma was based on a patient record of clinical features and diagnostic pulmonary function tests, corresponding with the Global Initiative for Asthma diagnostic criteria.27 Diagnosis of AERD was made on the basis of a history of wheezing or asthma attacks precipitated by nonsteroidal antiinflammatory drugs. The data of the presence of occupational exposure was available only in 107 (60.1%) patients. Of these, 34 (19.1%) had occupational airway exposure. The main substances causing job exposure were: bioaerosols, agricultural organic particles, wood/textile dust, mites, molds, flour, and reactive chemicals/metalwork. None of the subjects had undergone aspirin desensitization, allergen immunotherapy, or anti IgE therapy prior to operation or during follow‐up.
The information of other general diseases than CRS/AR/asthma was available from 173 patients (97.2%). Of these, there were no reported other diseases in 118 (68.2%) patients, and 55 (41.8%) of patients reported suffering from diseases (other than CRS/AR/asthma) with regular need of medications. Twenty‐one (12.1%) patients were reported as having one additional disease, 13 (7.5%) as having two diseases, and 21 (22.2%) as having three or more diseases. The most frequent diseases were (number of patients): heart and vascular diseases (40), nonasthma respiratory diseases (23), obesity/hyperlipidemia/diabetes (15), thyroid diseases (5), musculoskeletal diseases (5), and psychiatric diseases (5).
Data Analysis
Statistical analysis was carried out by the IBM SPSS Statistics 23 Statistical Software Package (SPSS Inc., Chicago, IL). Descriptive statistics for subject characteristics were presented in the CRS groups with/without revision surgery in 5 years. Fisher's exact test (2‐tailed) was used to compare patient characteristics. The Kruskal‐Wallis test and Mann Whitney U test was used to study the comparison of the total Lund‐Mackay scores of sinus computed tomography (CT) scans or magnetic resonance imaging (MRI). The time periods between the initial study operation and second surgeries were analyzed by the Kaplan‐Meier method and compared with the log‐rank test. Survival was calculated from the date of the initial study operation to first follow‐up revision sinus surgery and/or nasal polypectomy/death/end of March 2015, whichever came first. Univariate and multivariable Cox's proportional hazard models for interval to second surgery were constructed with the following predictor variables: age; gender; asthma diagnosis; AERD; AR; current/recurrent NP; previous sinus surgery and/or nasal polypectomy operation(s); smoking history; need for regular intranasal corticosteroids; preoperatively administered peroral corticosteroid course; and the initial study operation (only polypectomy, ESS ± polypectomy, only maxillary mini‐invasive ESS e.g. uncinectomy‐only on one side, only maxillary balloon sinuplasty). The variables for the multivariable Cox's proportional hazard model were chosen from those that were statistically significant variables in univariate Cox's proportional hazard models. Statistical significance was set at a P level of less than 0.05, and all tests were 2‐tailed.
RESULTS
Patient Characteristics
The characteristics are shown in Table 1. We compared the factors between the groups with/without revision surgery in 5 years. These patient groups were significantly different in terms of the presence of AR, need for regular intranasal corticosteroid therapy, and previous sinus surgery and/or nasal polypectomy (Table 1). Preoperative sinus CT scans only were available in 89 (50%) of the patients. Of these, the mean (minimum–maximum) Lund‐Mackay total score of CT scans or MRI was 7.9 (0–22) in the group that did not undergo revision surgery in 5 years, and was 8.7 (3–17) in the group that underwent revision surgery in 5 years. This difference was not statistically significant (P = 0.74 by Mann Whitney U test). Sinus surgery and/or nasal polypectomy prior to the initial study operation had been performed in 32 (18.0%) patients. Of these, 26 (26.8%) were in the CRSwNP group and six (7.5%) were in the CRSsNP group. The difference was statistically significant (P = 0.001, Fisher's exact test).
Table 1.
Characteristics of CRS Patients With/Without Status of Revision Sinus Surgery and/or Polypectomy 5 Years After Current Operation.
| Revision Sinus Surgery and/or Polypectomy in 5 Years | |||||
|---|---|---|---|---|---|
| No (N = 161) | Yes (N = 17) | ||||
| N | % | N | % | P Value | |
| Gender | |||||
| Male | 79 | 49.1 | 7 | 41.2 | .62 |
| Female | 82 | 50.9 | 10 | 58.8 | |
| Age | |||||
| < 40 years | 46 | 28.6 | 7 | 41.2 | .28 |
| ≥ 40 years | 115 | 71.4 | 10 | 58.8 | |
| Allergic rhinitis | |||||
| No | 101 | 62.7 | 4 | 23.5 | .002 |
| Yes | 56 | 34.8 | 13 | 76.5 | |
| Unknown | 4 | 2.5 | 0 | 0 | |
| Asthma | |||||
| No | 105 | 65.2 | 10 | 58.8 | .60 |
| Yes | 55 | 34.2 | 7 | 41.2 | |
| Unknown | 1 | 0.6 | 0 | 0 | |
| AERD | |||||
| No | 144 | 89.4 | 13 | 76.5 | .12 |
| Yes | 17 | 10.6 | 4 | 23.5 | |
| Current nasal polyps | |||||
| No | 77 | 47.8 | 4 | 23.5 | .11 |
| ACP | 7 | 4.3 | 1 | 4.9 | |
| Conventional NP | 77 | 47.8 | 12 | 70.6 | |
| Recurrent nasal polyps | |||||
| No | 149 | 92.5 | 13 | 76.5 | .05 |
| Yes | 12 | 7.5 | 4 | 23.5 | |
| Previous and/or current nasal polyps | |||||
| No or ACP | 84 | 52.2 | 5 | 29.4 | .06 |
| Yes | 77 | 47.8 | 12 | 70.6 | |
| Smokers | |||||
| No | 124 | 77.0 | 15 | 88.2 | .53 |
| Yes | 34 | 21.1 | 2 | 11.8 | |
| Unknown | 3 | 1.9 | 0 | 0 | |
| Need for regular intranasal corticosteroid | |||||
| No | 93 | 57.8 | 2 | 11.8 | <.001 |
| Yes | 59 | 36.6 | 15 | 88.2 | |
| Unknown | 9 | 5.6 | 0 | 0 | |
| Preoperative peroral corticosteroid | |||||
| No | 148 | 91.9 | 12 | 70.6 | .016 |
| Yes | 8 | 5.0 | 4 | 23.5 | |
| Unknown | 5 | 3.1 | 1 | 5.9 | |
| Previous sinus surgery and/or polypectomy | |||||
| No | 137 | 85.1 | 8 | 47.1 | .001 |
| Yes | 23 | 14.3 | 9 | 52.9 | |
| Unknown | 1 | 0.6 | 0 | 0 | |
| Current sinonasal operation | |||||
| Only polypectomy | 32 | 19.9 | 0 | 0 | .13 |
| ESS ± polypectomy | 82 | 50.9 | 11 | 64.7 | |
| Only maxill. mini‐invasive ESS 1 | 23 | 14.3 | 2 | 11.8 | |
| Only maxill. balloon sinuplasty | 24 | 14.9 | 4 | 23.5 | |
| Ethmoidectomy performed as a part of current surgery | |||||
| No | 109 | 67.7 | 6 | 35.3 | .010 |
| Yes | 52 | 32.3 | 11 | 64.7 | |
P values by Fisheŕs exact test.
1Uncinectomy‐only in one side and uncinectomy + antrostomy on the other side of each patient.
AERD = aspirin‐exacerbated respiratory disease; ACP = antrochoanal polyp; ESS = endoscopic sinus surgery; maxill. = maxillary (sinus); NP = nasal polyps.
Revision Operations During 5‐Year Follow‐Up
There were 17 (9.6%) CRS patients who had undergone at least one revision CRS operation in 5 years (Table 1). During the 5‐year follow‐up, the factors associating significantly with the need for revision sinus surgery and/or nasal polypectomy were AR, AERD, the need of regular intranasal corticosteroid treatment, preoperative peroral corticosteroid, previous sinus surgery and/or nasal polypectomy, and ethmoidectomy performed as part of current surgery (P < 0.02) (Table 1) (Fig. 1). In contrast, age, gender, smoking, method of the current operation, recurrent NP, and the presence of asthma or NP (current ± previous) did not associate with the revision during follow‐up (P ≥ 0.05) (Table 1). Occupational exposure, the presence of general diseases, or the number of general diseases (other than CRS/AR/asthma) were not associated with the revision during follow‐up (P > 0.05) .
Figure 1.

Comparison of number of revision sinus operations and/or revision nasal polypectomies in different CRS patient groups during follow‐up of in average 9 years. CRS patient group (A) with/without the presence of allergic rhinitis. (B) The presence of aspirin‐exacerbated respiratory disease. (C) The regular use of intranasal corticosteroid. (D) Preoperative peroral corticosteroid course. (E) Recurrent nasal polyps. (F) Previous sinus surgery and/or nasal polypectomy.
P values by Fisheŕs exact test.
AERD = aspirin‐exacerbated respiratory disease; CRS = chronic rhinosinusitis.
Revision Operations During Total Follow‐up Time
The mean (minimum–maximum) total follow‐up of all subjects was 8.7 (1–14) years. There were eight deaths (6 CRSwNP, 2 CRSsNP) during follow‐up time. None of the deaths were caused by CRS or surgery of CRS. None had undergone revision of CRS during follow‐up prior to death. The total follow‐up of the patients who were alive until 2015 was 8.9 (4–14) years. During the total follow‐up, 17 (9.6%) of the CRS patients had undergone one revision operation; three (1.7%) patients had undergone two revisions; and three (1.7%) patients had undergone three revisions. The revision operations were ethmoidectomy (N = 14), nasal polypectomy (N = 7), middle meatal antrostomy (N = 12), and frontal recess/sinus surgery (N = 3). Moreover, septoplasty was performed to four patients who also underwent CRS surgery. The revision operation was more extensive than the previous one in 20 (87.0%) of the cases. The number of revision operations during follow‐up was significantly higher in patients with reported AR, AERD, regular need for intranasal corticosteroid, or with preoperative peroral corticosteroid treatment (P < 0.004) (Fig. 1). In contrast, the following factors did not associate with the revision during follow‐up: age; gender; smoking; occupational exposure; method of the current operation; and the presence of asthma or NP, the presence of general diseases (other than CRS/AR/asthma), or the number of general diseases (P > 0.05, Fisher's exact test).
Kaplan Meier Survival Analyses
In the survival analyses, the mean (minimum–maximum) follow‐up time was 7.9 (1–14) years until the first revision surgery/death/end of March 2015, whichever came first. The factors associating significantly with the need for revision sinus surgery and/or nasal polypectomy were AR, AERD, recurrent NP, the need for regular intranasal corticosteroid treatment, preoperative peroral corticosteroid, and previous sinus surgery and/or nasal polypectomy (P < 0.02) (Fig. 2). However, age, gender, smoking, method of the current operation, or the presence of asthma or NP did not associate with the revision during follow‐up (P > 0.05) (Table 2). The presence of occupational exposure or the presence of other general diseases than CRS/AR/asthma did not associate with the need for revision surgery (P = 0.90, P = 0.27, respectively, by the log‐rank test).
Figure 2.
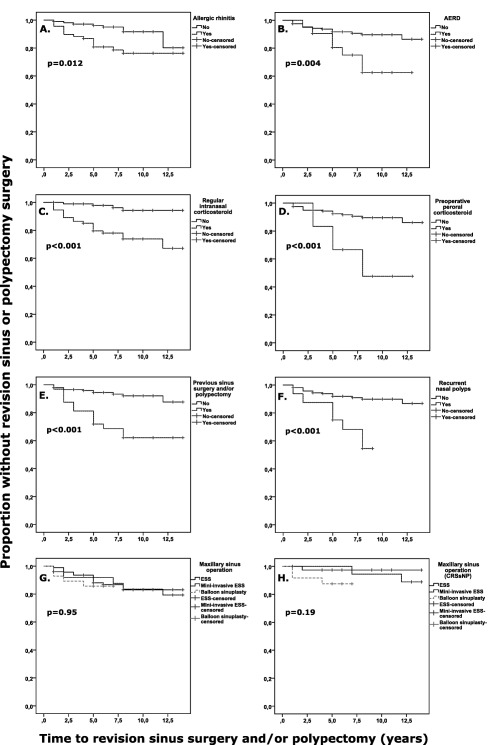
Predictive effect of different factors to the time until revision sinus surgery and/or nasal polypectomy was performed according to the Kaplan‐Meier method. (A) Predictive effect of a patient history of doctor‐diagnosed allergic rhinitis in all CRS patients (N = 174). (B) Predictive effect of a patient history of doctor‐diagnosed AERD in all CRS patients (N = 178). (C) Predictive effect patient‐reported regular need of intranasal corticosteroid treatment in all CRS patients (N = 169). (D) Predictive effect of preoperative peroral corticosteroid treatment in all CRS patients (N = 172). (E) Predictive effect of previous sinus surgery and/or nasal polypectyomy in all CRS patients (N = 177). (F) Predictive effect of recurrent nasal polyps in all CRS patients (N = 178). (G) Predictive effect of maxillary sinus operation technique in all the CRS patients who underwent current maxillary sinus surgery (N = 146). (H) Predictive effect of maxillary sinus operation technique in the subgroup of CRSsNP patients who underwent current maxillary sinus surgery (N = 81).
P values by log‐rank test.
AERD = aspirin‐exacerbated respiratory disease; CRS = chronic rhinosinusitis; CRSsNP = chronic rhinosinusitis without nasal polyps; ESS = endoscopic sinus surgery; mini‐invasive ESS = uncinectomy‐only in one side and uncinectomy + antrostomy on the other side of each patient.
Table 2.
Unadjusted and Adjusted Cox's Proportional Hazard Models for Variables Analyzed Fitted for Need for Revision Sinus Surgery and/or Nasal Polypectomy During Follow‐up of Average 9 Years.
| Revision Sinus Surgery and/or Polypectomy | |||||||
|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | ||||||
| Events (23) | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Gender | |||||||
| Male | 9 | 1 | Not entered | ||||
| Female | 14 | 1.54 | 0.67–3.56 | .31 | |||
| Age | |||||||
| < 40 years | 8 | 1 | Not entered | ||||
| ≥ 40 years | 15 | 0.77 | 0.33–1.82 | .56 | |||
| Allergic rhinitis | |||||||
| No | 8 | 1 | 1 | ||||
| Yes | 15 | 2.86 | 1.21–6.76 | .017 | 3.97 | 1.34–11.73 | .013 |
| Asthma | |||||||
| No | 12 | 1 | Not entered | ||||
| Yes | 11 | 1.71 | 0.76–3.88 | .20 | |||
| AERD | |||||||
| No | 16 | 1 | 1 | ||||
| Yes | 7 | 3.37 | 1.38–8.23 | .008 | 0.34 | 0.076–1.48 | .15 |
| Previous and/or current nasal polyps | |||||||
| No or ACP1 | 7 | 1 | Not entered | ||||
| Yes | 16 | 2.31 | 0.94–5.68 | .07 | |||
| Recurrent nasal polyps | |||||||
| No | 16 | 1 | 1 | ||||
| Yes | 7 | 5.08 | 2.07–12.45 | < .001 | 5.77 | 1.37–24.3 | .017 |
| Smokers | |||||||
| No | 21 | 1 | Not entered | ||||
| Yes | 2 | 0.34 | 0.08–1.45 | .15 | |||
| Need for regular intranasal corticosteroid | |||||||
| No | 4 | 1 | 1 | ||||
| Yes | 19 | 6.63 | 2.26–19.49 | .001 | 4.64 | 1.47–14.68 | .009 |
| Preoperative peroral corticosteroid | |||||||
| No | 16 | 1 | 1 | ||||
| Yes | 6 | 5.13 | 2.00–13.15 | .001 | 9.27 | 1.89–45.40 | .006 |
| Previous sinus surgery and/or nasal polypectomy | |||||||
| No | 11 | 1 | 1 | ||||
| Yes | 12 | 5.06 | 2.23–11.49 | <.001 | 2.50 | 0.60–10.36 | .21 |
| Ethmoidectomy performed as a part of current surgery | |||||||
| No | 9 | 1 | 1 | ||||
| Yes | 14 | 2.70 | 1.17–6.27 | .021 | 0.48 | 0.13–1.80 | .28 |
The six variables that entered the adjusted Cox's proportional hazard were chosen from those that were statistically significant variables in unadjusted Cox's proportional hazard models.
1When removing ACP from the analysis, the result remained similar.
ACP = antrochoanal polyp; AERD = aspirin‐exacerbated respiratory disease; CI = confidential interval; HR = hazard ratio.
Cox Regression Analyses
Cox's proportional hazards analysis was conducted to determine which factors were predictive of surgery recurrence. Of the possible predictive factors, AR, AERD, recurrent NP, the need of regular intranasal corticosteroid treatment, preoperative peroral corticosteroid, previous sinus surgery and/or polypectomy, and ethmoidectomy performed as a part of current surgery were significantly predictive for revision surgery (P < 0.03) (Table 2). No other covariate was significantly predictive surgery recurrence. Antrochoanal polyps did not affect the result (Table 2). Each of the previously mentioned seven significant predictive variables was entered into the adjusted Cox's proportional hazard model. In this multivariable analysis, AR, peroral or intranasal corticosteroid treatment, and recurrent NP were significantly predictive for surgery recurrence (Table 2). Thus AR, corticosteroid, treatment and recurrent NP seems to predict independently surgery recurrence.
DISCUSSION
The clinically most useful predictive factors would be specific, sensitive, easy, and usable across nonselected CRS populations. Thus, this study was implemented to evaluate simple factors associating the revision rate of heterogeneous CRS population with both CRSsNP and CRSwNP phenotypes and with inclusion of also less severe cases. The most significant finding was that the factors associating significantly with the recurrence of surgery were AR, recurrent NP, peroral or intranasal corticosteroid treatment, and previous surgery. These factors associated with the number of revision operations, and they also independently predicted the need for revision surgery in the survival analyses.
To the knowledge of the authors, there exist only two previous studies on recurrent sinus surgery using survival analysis technique. Mendelsohn et al. performed a survival analysis of a cohort of 549 CRSwNP patients who underwent ESS over a 10‐year period.16 Revision surgery occurred at a high rate, especially in patients with asthma, AERD, or frontal sinus disease. In our study population of both CRSsNP and CRSwNP patients, we were able to demonstrated association between recurrent NP and previous operation(s); however, we were not able to demonstrate independent association of asthma or AERD with recurrent surgery. This might reflect the differences in our study populations. Wu et al. reviewed records of 299 CRSwNP patients who underwent two or more surgeries from 1987 through 2011.17 They found that revision surgery and regrowth of nasal polyps appears to be affected by smoking and operative technique but not by other factors such as asthma or advanced CT stage. The performance of middle turbinate resection in this study population was found to significantly delay the time until revision surgery; however, the beneficial effect of turbinectomy appeared to dissipate by 8 years. In our study, smoking did not predict surgery recurrence; therefore, further studies on this are required.
Wu et al. found that the mean number of surgeries performed per CRSwNP patient was 2.3, and the mean time between sinus surgeries was 4.9 years.17 Contrasted to this, in our study the median number of revision surgeries during follow‐up was 0 in both CRSsNP and CRSwNP groups. However, we found that the higher number of revision surgeries associated with the history of AR, AERD, recurrent NP, previous sinus surgery and/or nasal polypectomy, and the regular need for intranasal corticosteroid and preoperative corticosteroid, which reflects the previous findings.17
More studies exist on revision rate, especially in CRSwNP patients without using survival method. A study showed that male patients and younger adult patients were found to have higher revision rates13; however, we were not able to demonstrate this. In addition, Chang et al. found that patients with asthma or allergic rhinitis had higher revision rates compared with those patients without these comorbidities.13 This is in part in line with our findings that allergic rhinitis independently predicted recurrent surgery. The etiology between allergy and rhinosinusitis is multifactorial, and a clear consensus of their relation does not exist at present. However, previous studies suggest atopy being a predisposing factor for CRS. It has been postulated that patients with allergic rhinitis have edematous nasal mucosa, impaired cilia function, and overproduction of secretions, which lead to blockage at the site of ostiomeatal complex, impaired drainage and ventilation, and again to sinus problems.28 Recently, Wilson et al. reviewed 24 articles examining the relationship of allergy and CRSsNP and/or CRSwNP.29 According to their analysis, the role of allergy in CRSwNP and CRSsNP continues to be controversial, with the level of evidence poor.29 We recently demonstrated that sensitization to over five common airborne allergens and to several allergen groups associate strongly with adult asthma.30 It could be speculated that multiple allergen sensitization might be associated with progressing CRS, whereas simple pollen allergic rhinitis does not associate with severe CRS. Although not yet proven, this could in part explain controversial findings of the association between CRS(wNP) and atopy. Thus, future studies on sensitization patterns and the association with CRS progression would be important to understand the mechanisms behind CRS development, prevention of CRS, and its progression.
The presence of general diseases did not associate with recurrent surgery. To the knowledge of the authors, there are no reports of the effect of general diseases on the recurrence of CRS surgery. The effect of occupational exposure has previously been studied. Hox et al. performed a questionnaire study with 890 patients who had undergone one or more ESS, and with 182 controls.18 After adjustments, the authors detected that the prevalence of occupational exposures associated significantly with the number of ESS procedures. The authors concluded that exposure at work appears to be a risk factor for the occurrence of CRS and for its recurrence or persistence, as evidenced by the need for revision surgery.18 In this current study, we were not able to comprehensively study the effect of occupational exposure to revision rate because the occupational data was available from only about 40% of the patients.
Performing ethmoidectomy as a part of current surgery was associated with recurrent CRS surgery. After adjustment, ethmoidectomy did not associate with revision surgery. This might be due to the fact that the majority of those who underwent primary ethmoidectomy were NP patients, and the status of recurrent NP had stronger independent association with the need for revision surgery than ethmoidectomy.
A study suggests that primary ESS failure is most often associated with re‐obstruction in the area of the ostiomeatal complex.13 We demonstrated here that a minimally invasive operation slightly and insignificantly increased the revision rate in the CRSsNP subgroup; however, minimally invasive approaches (e.g., balloon sinuplasty and uncinectomy‐only) did not have an effect on the revision rate when observing all CRS patients. In terms of the need for recurrent CRS surgery, a minimally invasive technique might be sufficient; however, our previous controlled study demonstrates that radiological recovery was slightly inferior after uncinectomy‐only technique compared to antrostomy.25 Moreover, it is highly important to further follow up the recurrence of surgeries in patients with balloon sinuplasty because the balloon sinuplasty procedures were started in Finland in 2008.
We found that previous surgery was performed in about 26% of CRSwNP patients and to a significantly lower extent in CRSsNP patients (7.5%). In accordance with our findings, a study observed a revision rate in CRS 4,484 patients who had undergone ESS.13 The study group found that the number of patients who underwent revision ESS was 528 (11.78%). Another study group investigated 1,249 CRS patients and 221 controls in United Kingdom by means of a self‐administered questionnaire.2 A total of 396 (57%) patients with CRSwNPs/allergic fungal rhinosinusitis (AFRS) reported having undergone previous endoscopic nasal polypectomy; of those, 182 of the 396 (46%) reported having received more than one operation.2 In comparison, surgical rates in patients with CRSsNPs were significantly lower: 13% of cases specifically reported ESS; and of those, only 30% reported multiple procedures.2 The reasons of higher percentage of previous surgery in the study of Philpott et al. compared to ours might be related to differences in the populations, study design, and management of CRS patients.2
We found that only about one‐tenth of CRS patients required re‐operation in 5 years. A study showed that revision surgery rates at 5 years were 10%, 25%, and 37% for control patients, patients with asthma, and patients with AERD, respectively.16 Prior studies have reported recurrence rates in shorter mean follow‐up periods (less than 5 years) of between 21% and 66% for CRSwNP, but most did not subdivide among patient groupings or did not comment on revision surgery rates.16, 31, 32, 33, 34, 35, 36, 37
Philpott et al. showed the highest rate of revision surgery to be among those with CRSwNPs and AFRS, with rates of previous surgery almost twice that of those without nasal polyps, which is supported by the U.K. Sinonasal Audit.2 We also demonstrated here a clear association of CRSwNP phenotype with both increased prior operation(s), as well as recurrent NP as an independent predictor for revision during follow‐up. There is a growing acceptance that patients with and without polyps have distinct differences. This is reflected in the current iteration of EPOS, with different treatment algorithms for the two main phenotypes.1, 2 In line with this, we showed that recurrent NP was an independent predictive factor for recurrence of surgery.
Studies suggest that a crucial factor in the success or failure of surgical intervention will be patient compliance with ongoing medical management postoperatively.2 The authors suggested that this may currently be counteracted by differing advice from primary and secondary care practitioners and emphasizes the need for greater awareness of guidelines; however, there is also a need for further clinical trials in terms of medical treatment to underpin this.2 We found that regular intranasal corticosteroids did not delay recurrence of surgery; in contrast, they associated with increased revision rate. Regular intranasal corticosteroid treatment, which most patients reported to continue to use regularly also postoperatively, predicted independently increased revision rate. Moreover, the need for preoperative peroral corticosteroid course predicted independently increased revision rate. The need for regular intranasal and preoperative peroral corticosteroids would putatively reflect severe CRSwNP and uncontrolled CRS (with/without atopy). Together, it seems that a more severe/uncontrolled CRS phenotype could be identified. It might be related to recurrent NP and atopy but might be independent of AERD and asthma. Despite maximum management, this phenotype might need frequent surgical interventions. The putative reasons for progression of CRS in these patients might be genetic and immunological, affecting interactions between host barrier immunity and environmental irritants/microbes.7 These patients do not fully respond the current treatment with corticosteroids and surgery, and are thus in high need for advanced management such as biological treatment and other future therapeutic interventions.
This study did not measure polyp regrowth or other signs of uncontrolled CRS, which would have reflected more precisely the progressing CRS. Revision CRS surgery was chosen as the end point because there is a lack of good objective data of CRS progression. Serial nasal endoscopy shows nasal polyp regrowth but has limited correlation with the severity of CRS(sNP)38; and serial sinus CT scans cannot be performed due to radiation. Thus, we observed the recurrent surgery as a clearly defined event, which indirectly reflects to CRS progression. This study did not distinguish between revision CRS surgery due to suboptimal surgery and to recurrence of CRS. This study also did not measure differences in postoperative medical treatments that could affect timing of revision surgery. Our scope was to detect predictive factors that would associate with recurrent CRS surgery independent of these factors and despite heterogeneity in CRS population.
This study has several limitations. The sample was heterogeneous and small in size for clinically relevant conclusions. No CRS cases with cystic fibrosis or with primary ciliary dyskinesia were available for this study. A shortcoming is that the diagnosis of AR was not based on skin‐prick test positivity in all patients; and the diagnosis of AERD was not based on nonsteroidal anti‐inflammatory drugs challenge test in all patients. There also might have been limitations in the collection of other variables from patient records. The fact that patients with balloon sinuplasty were followed up for a significantly shorter time than other patients introduces the possibility that the increased revision surgery rates were not yet detected properly in this group. Our analysis of revision surgery may have been influenced by several factors unrelated to recurrence of CRS, including wait times for surgery and patients' preferences to delay surgery for personal reasons. Other factors that can also affect the timing of revision surgery include the patient's tolerance of recurrent sinusitis symptoms, the operative technique used at the time of the initial surgery, and the surgeon's opinion concerning when revision surgery is clinically warranted. Additionally, some patients might have sought treatment elsewhere and were lost to follow‐up. A strength of our study was our observation of the heterogeneous CRS population and not only the CRSwNP phenotype.
CONCLUSION
This study demonstrate that revision surgery occurs in about 10% of CRS patients despite active therapy. Progressive chronic inflammatory phenotype would putatively be recognized by relatively simple questions concerning atopy, recurrent NP, previous CRS surgeries, and need for corticosteroid therapy. These patients should be informed of the significant likelihood of revision surgery. Further studies with increased number of patients, populations, and rare cases are mandatory to evaluate whether these questions and factors would be relevant for developing better detection and management of uncontrolled CRS.
Acknowledgment
We thank Marja‐Leena Oksanen, Arto Ranta, Miia Seppälä, Juha Silvola, Mikko Suvinen, and Tommi Torkkeli for their excellent assistance in this study.
Funding: The study was supported in part by the Competitive Research Funding of the Tampere Medical Research Fund of Tampere University Hospital, and in part by research grants from the Finnish Medical Foundation, Finnish Association of Otorhinolaryngology and Head and Neck Surgery, Finnish Society of Allergology and Immunology, Jane and Aatos Erkko Foundation, Väinö and Laina Kivi Foundation, and Yrjö Jahnsson Foundation.
BIBLIOGRAPHY
- 1. Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl 2012;3 p preceding table of contents, 1–298. [PubMed] [Google Scholar]
- 2. Philpott C, Hopkins C, Erskine S, et al. The burden of revision sinonasal surgery in the UK‐data from the Chronic Rhinosinusitis Epidemiology Study (CRES): a cross‐sectional study. BMJ Open 2015;5:e006680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hastan D, Fokkens WJ, Bachert C, et al. Chronic rhinosinusitis in Europe—an underestimated disease. A GA(2)LEN study. Allergy 2011;66:1216–1223. [DOI] [PubMed] [Google Scholar]
- 4. Kim YS, Kim NH, Seong SY, Kim KR, Lee GB, Kim KS. Prevalence and risk factors of chronic rhinosinusitis in Korea. Am J Rhinol Allergy 2011;25:117–121. [DOI] [PubMed] [Google Scholar]
- 5. Pilan RR, Pinna FR, Bezerra TF, et al. Prevalence of chronic rhinosinusitis in Sao Paulo. Rhinology 2012;50:129–138. [DOI] [PubMed] [Google Scholar]
- 6. Smith KA, Orlandi RR, Rudmik L. Cost of adult chronic rhinosinusitis: a systematic review. Laryngoscope 2015;125:1547–1556. [DOI] [PubMed] [Google Scholar]
- 7. Toppila‐Salmi S, van Drunen CM, Fokkens WJ, et al. Molecular mechanisms of nasal epithelium in rhinitis and rhinosinusitis. Curr Allergy Asthma Rep 2015;15:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang JE, White A, Simon RA, Stevenson DD. Aspirin‐exacerbated respiratory disease: burden of disease. Allergy Asthma Proc 2012;33:117–121. [DOI] [PubMed] [Google Scholar]
- 9. Makowska J, Lewandowska‐Polak A, Kowalski ML. Hypersensitivity to aspirin and other NSAIDs: diagnostic approach in patients with chronic rhinosinusitis. Curr Allergy Asthma Rep 2015;15:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khalil HS, Nunez DA. Functional endoscopic sinus surgery for chronic rhinosinusitis. Cochrane Database Syst Rev 2006;CD004458. [DOI] [PubMed] [Google Scholar]
- 11. DeConde AS, Bodner TE, Mace JC, Smith TL. Response shift in quality of life after endoscopic sinus surgery for chronic rhinosinusitis. JAMA Otolaryngol Head Neck Surg 2014;140:712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rudmik L, Mace J, Soler ZM, Smith TL. Long‐term utility outcomes in patients undergoing endoscopic sinus surgery. Laryngoscope 2014;124:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang CC, Tai CJ, Ng TY, Tsou YA, Tsai MH. Can FESS combined with submucosal resection (SMR)/septoplasty reduce revision rate? Otolaryngol Head Neck Surg 2014;151:700–705. [DOI] [PubMed] [Google Scholar]
- 14. Bhattacharyya N. Clinical outcomes after revision endoscopic sinus surgery. Arch Otolaryngol Head Neck Surg 2004;130:975–978. [DOI] [PubMed] [Google Scholar]
- 15. Hopkins C, Slack R, Lund V, Brown P, Copley L, Browne J. Long‐term outcomes from the English national comparative audit of surgery for nasal polyposis and chronic rhinosinusitis. Laryngoscope 2009;119:2459–2465. [DOI] [PubMed] [Google Scholar]
- 16. Mendelsohn D, Jeremic G, Wright ED, Rotenberg BW. Revision rates after endoscopic sinus surgery: a recurrence analysis. Ann Otol Rhinol Laryngol 2011;120:162–166. [DOI] [PubMed] [Google Scholar]
- 17. Wu AW, Ting JY, Platt MP, Tierney HT, Metson R. Factors affecting time to revision sinus surgery for nasal polyps: a 25‐year experience. Laryngoscope 2014;124:29–33. [DOI] [PubMed] [Google Scholar]
- 18. Hox V, Delrue S, Scheers H, et al. Negative impact of occupational exposure on surgical outcome in patients with rhinosinusitis. Allergy 2012;67:560–565. [DOI] [PubMed] [Google Scholar]
- 19. Albu S, Tomescu E, Mexca Z, Nistor S, Necula S, Cozlean A. Recurrence rates in endonasal surgery for polyposis. Acta Otorhinolaryngol Belg 2004;58:79–86. [PubMed] [Google Scholar]
- 20. Friedman WH, Katsantonis GP. Intranasal and transantral ethmoidectomy: a 20‐year experience. Laryngoscope 1990;100:343–348. [DOI] [PubMed] [Google Scholar]
- 21. Wynn R, Har‐El G. Recurrence rates after endoscopic sinus surgery for massive sinus polyposis. Laryngoscope 2004;114:811–813. [DOI] [PubMed] [Google Scholar]
- 22. Lawson W. The intranasal ethmoidectomy: an experience with 1,077 procedures. Laryngoscope 1991;101:367–371. [DOI] [PubMed] [Google Scholar]
- 23. Honkanen T, Luukkainen A, Lehtonen M, et al. Indoleamine 2,3‐dioxygenase expression is associated with chronic rhinosinusitis with nasal polyps and antrochoanal polyps. Rhinology 2011;49:356–363. [DOI] [PubMed] [Google Scholar]
- 24. Koskinen A, Penttila M, Myller J, et al. Endoscopic sinus surgery might reduce exacerbations and symptoms more than balloon sinuplasty. Am J Rhinol Allergy 2012;26:e150–e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Myller J, Dastidar P, Torkkeli T, Rautiainen M, Toppila‐Salmi S. Computed tomography findings after endoscopic sinus surgery with preserving or enlarging maxillary sinus ostium surgery. Rhinology 2011;49:438–444. [DOI] [PubMed] [Google Scholar]
- 26. Toppila‐Salmi SK, Myller JP, Torkkeli TV, et al. Endothelial L‐selectin ligands in sinus mucosa during chronic maxillary rhinosinusitis. Am J Respir Crit Care Med 2005;171:1350–1357. [DOI] [PubMed] [Google Scholar]
- 27. Reddel HK, Bateman ED, Becker A, et al. A summary of the new GINA strategy: a roadmap to asthma control. Eur Respir J 2015;46:622–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feng CH, Miller MD, Simon RA. The united allergic airway: connections between allergic rhinitis, asthma, and chronic sinusitis. Am J Rhinol Allergy 2012;26:187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wilson KF, McMains KC, Orlandi RR. The association between allergy and chronic rhinosinusitis with and without nasal polyps: an evidence‐based review with recommendations. Int Forum Allergy Rhinol 2014;4:93–103. [DOI] [PubMed] [Google Scholar]
- 30. Toppila‐Salmi S, Huhtala H, Karjalainen J, et al. Sensitization pattern affects the asthma risk in Finnish adult population. Allergy 2015;70:1112–1120. [DOI] [PubMed] [Google Scholar]
- 31. Jantti‐Alanko S, Holopainen E, Malmberg H. Recurrence of nasal polyps after surgical treatment. Rhinol Suppl 1989;8:59–64. [PubMed] [Google Scholar]
- 32. Schaitkin B, May M, Shapiro A, Fucci M, Mester SJ. Endoscopic sinus surgery: 4‐year follow‐up on the first 100 patients. Laryngoscope 1993;103:1117–1120. [DOI] [PubMed] [Google Scholar]
- 33. Smith TL, Mendolia‐Loffredo S, Loehrl TA, Sparapani R, Laud PW, Nattinger AB. Predictive factors and outcomes in endoscopic sinus surgery for chronic rhinosinusitis. Laryngoscope 2005;115:2199–2205. [DOI] [PubMed] [Google Scholar]
- 34. Kennedy DW. Prognostic factors, outcomes and staging in ethmoid sinus surgery. Laryngoscope 1992;102:1–18. [PubMed] [Google Scholar]
- 35. Kim JE, Kountakis SE. The prevalence of Samter's triad in patients undergoing functional endoscopic sinus surgery. Ear Nose Throat J 2007;86:396–399. [PubMed] [Google Scholar]
- 36. Drake‐Lee AB, Lowe D, Swanston A, Grace A. Clinical profile and recurrence of nasal polyps. J Laryngol Otol 1984;98:783–793. [DOI] [PubMed] [Google Scholar]
- 37. Vento SI, Ertama LO, Hytonen ML, Wolff CH, Malmberg CH. Nasal polyposis: clinical course during 20 years. Ann Allergy Asthma Immunol 2000;85:209–214. [DOI] [PubMed] [Google Scholar]
- 38. Ryan WR, Ramachandra T, Hwang PH. Correlations between symptoms, nasal endoscopy, and in‐office computed tomography in post‐surgical chronic rhinosinusitis patients. Laryngoscope 2011;121:674–678. [DOI] [PubMed] [Google Scholar]


