Abstract
In the last decade, many studies have shown that hydrogen gas or hydrogen water can reduce the levels of reactive oxygen species in the living body. Molecular hydrogen has antioxidant and antiapoptotic effects and a preventive effect on oxidative stress-induced cell death. In the present study, we investigated solidified hydrogen-occluding-silica (H2-silica) that can release molecular hydrogen into cell culture medium because the use of hydrogen gas has strict handling limitations in hospital and medical facilities and laboratories, owing to its physicochemical characteristics. Human esophageal squamous cell carcinoma (KYSE-70) cells and normal human esophageal epithelial cells (HEEpiCs) were used to investigate the effects of H2-silica on cell viability and proliferation. Cell migration was examined with wound healing and culture-insert migration assays. The intracellular levels of reactive oxygen species were evaluated with a nitroblue tetrazolium assay. To assess the apoptotic status of the cells, the Bax/Bcl-2 ratio and cleaved caspase-3 were analyzed by western blot. The results showed that KYSE-70 cells and HEEpiCs were generally inhibited by H2-silica administration, and there was a significant proliferation-inhibitory effect in an H2-silica concentration-dependent manner compared with the control group (P < 0.05) in KYSE-70. Apoptosis-inducing effect on KYSE-70 cells was observed in 10, 300, 600, and 1,200 ppm H2-silica, and only 1,200 ppm H2-silica caused a 2.4-fold increase in apoptosis in HEEpiCs compared with the control group as the index of Bax/Bcl-2. H2 silica inhibited cell migration in KYSE-70 cells, and high concentrations had a cytotoxic effect on normal cells. These findings should provide insights into the mechanism of inhibition of H2-silica on human cancer cells in vitro.
Keywords: molecular hydrogen, hydrogen-occluding-silica, cell migration, apoptosis-inducing effect, reactive oxygen species, car-cinogenic cells, normal human culture cells, cytotoxic effect
INTRODUCTION
It is well known that hydrogen is the most abundant and simplest chemical element in the universe and in our living body, and molecular hydrogen (H2) is a colorless, odorless, nonmetallic, tasteless, and highly flammable diatomic gas. The relative low solubility of hydrogen means that it has long been thought that it could not be absorbed into the body, and therefore, it was classified as an inert gas.1,2
In 2007, Ohsawa et al.3 found that hydrogen not only has antioxidant and antiapoptotic effects but also has a preventive effect on oxidative stress-induced apoptosis and a scavenge ability against intracellular hydroxyl radicals. Since then, many basic studies have demonstrated that H2 has a role as a novel antioxidant, and it is widely used for preventing and treating various diseases.4,5,6 The studies suggest that whether using inhaled H2 gas or oral H2 water, H2 is still an effective method for scavenging reactive oxygen species (ROS).3,7,8
In recent years, improvements in the therapeutic effects of chemotherapy for cancer have been required because of the wide variety of side effects. These side effects decrease the quality of life of patients and sometimes cause death. Ideally, an anticancer drug would not have any serious side effects and would have a selective effect, minimizing cytotoxicity to surrounding normal cells.
Therefore, the present study investigated hydrogen-occluding-silica (H2-silica), which is known to be a novel antioxidant and harmless particle that is blended with nano-sized silica and trace amounts of mineral. It consists of silicon dioxide (silica), potassium citrate, potassium carbonate, and magnesium sulfate. With regard to its antioxidant effects, H2-silica generates large amounts of hydrogen when it comes in contact with liquid, resulting in the neutralization of ROS.9,10,11 This reagent is expected to prevent aging and treat various diseases and further, such as to kill cancer cells.12
This study aimed to explore whether hydrogen-occluding-silica (H2-silica) has anticancer and apoptosis-inducing effects in human esophageal squamous cell carcinoma (KYSE-70) cells compared with normal human esophageal epithelial cells (HEEpiCs). Furthermore, cell migration was examined in both cell lines to investigate whether H2 could have a potency of inhibiting invasion by cancer cells and could therefore play a selective role in the protection of normal cells in vitro.
MATERIALS AND METHODS
Cell culture
The human esophageal squamous cell carcinoma KYSE-70 cell line was kindly provided from the Human Science Foundation (Osaka, Japan). The normal human esophageal epithelial cell (HEEpiC) line was purchased from ScienCell Research Laboratories (CA, USA) via Cosmo Bio Co., Ltd. (Tokyo, Japan).
KYSE-70 cells were routinely cultivated in Dulbecco’s modified Eagle’s minimum essential medium (DMEM; Wako Pure Chemical Industries, Ltd., Osaka, Japan) supplemented with 10% fetal bovine serum (FBS); Invitrogen Corp., CA, USA), 1% L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Wako Pure Chemical Industries, Ltd., Osaka, Japan). HEEpiCs were grown in Epithelial Cell Medium-2 (EpiCM-2, ScienCell, CA, USA), in accordance with the manufacturer’s instructions. Both cells were cultured to 80% confluence in a humidified atmosphere of 5% carbon dioxide in air at 37°C.
Once the cells were at 80% confluence under an inverted microscope (TCM400FLR, Labo America Inc., CA, USA), they were passaged with 0.25% (w/v) trypsin and 0.03% (w/v) trypsin inhibitor (Gibco, Darmstadt, Germany). In addition, the cells were split or re-fed with fresh medium every 3–5 days, depending on growth status. Both cell lines were seeded at a density of 2.0 × 105 cells per well in 6-well plates (BD Biosciences, NJ, USA) for each assay. Cell density was changed in accordance with well numbers of the microplates used in the experiments.
Preparation and administration of H2-silica
H2-silica was manufactured as silica hydride “Microcluster®,” kindly supplied from New-1-Ten-Rin Enterprise Co. Ltd. (Changhua County, Taiwan, China) via the Japanese Center of AntiAging MedSciences (Hiroshima, Japan). In advance of the administration for H2-silica, the basic parameters such as pH, temperature, and oxidization reduction potential (ORP) were measured (Figure 1). DMEM and EpiCM-2 were prepared containing H2-silica at 10, 100, 300, 600, 900, and 1,200 ppm and applied to the KYSE-70 cells and HEEpiCs that were incubated for 48 and 96 hours, respectively.
Figure 1.
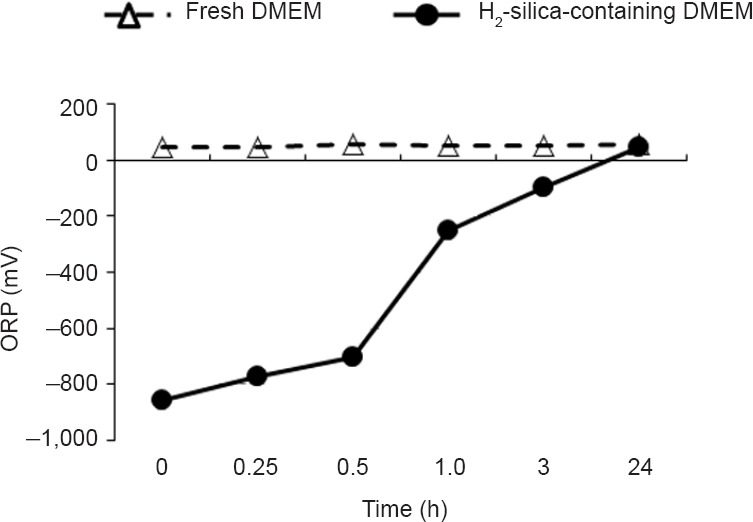
The oxidation–reduction potential of DMEM containing H2-silica.
Note: The black cycle indicates DMEM containing H2-silica. The open triangle indicates fresh DMEM without H2-silica. ORP: Oxidization reduction potential; h: hour(s); H2-silica: hydrogen-occluding-silica.
4-[3-(2-methoxy-4-nitro-phenyl)-2-[4-nitrophenyl]-2H-5-tetrazolio]-1,3-benzene disulfonate sodium salt (WST-8) assay
Quantification of cell proliferation, growth, viability, and population was evaluated spectrophotometrically by WST-8 assay (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s instructions and in accordance with our previous study.13
The medium from the KYSE-70 cells and HEEpiCs cultures was removed and cells were transferred to a 96-well microplate (Outlier, OR, USA), and a mixture of 10 μL of CCK-8 (Dojindo Laboratories) and 100 μL of either DMEM or EpiCM-2 were added to each well, and incubated for approximately 1 hour. The resultant diformazan formation was measured spectrophotometrically at a wavelength of 450 nm with a microplate reader (CHROMATE 4300, Awareness Technology Inc., FL, USA).
Wound-healing assay
Cell migration was assessed in a classic wound-healing assay.14,15 First, the tip of a 200 μL micropipette was used to make a straight scratch on a confluent monolayer of cells in a 6-well plate in order to simulate a wound. The cells were then rinsed with Dulbecco’s phosphate-buffered saline (D-PBS) (-) before serum-free DMEM-or EpiCM-2 containing H2-silica and penicillin/streptomycin and L-glutamine were added. Scratches were made with the pipette tip at an angle of around 30° to keep the scratch width limited. This allowed imaging of both wound edges using the 40× objective lens of a microscope. After 12 hours incubation at 37°C, wound closure in the area was photographed using an inverted phase microscope (CK2, Olympus, Tokyo, Japan) at 40× magnification. The wound closure areas were randomly selected and calculated (mm2) in order to show the closure of the wounds at each time period.
In addition, a culture-insert migration assay was also used to observe the inhibitory effect of H2-silica administration on cell migration and invasion of cells. In accordance with the manufacturer’s instructions, a culture insert (ibidi GmbH, Martinsried, Germany) was placed into a 35-mm tissue culture dish (Corning International, Inc.). Cell suspensions were prepared at 1–2 × 105 cells/mL in medium, and 70 μL was transferred to each chamber of the culture insert. Once the cells grew to ~90% confluence, the insert was removed and H2-silica-containing media were added at different concentrations (0, 10, 100, 300, 600, and 1,200 ppm). The methods of image and calculation were mentioned above.
Nitroblue tetrazolium (NBT) assay
Measurements of intracellular ROS levels in KYSE-70 cells and HEEpiCs were made using the NBT assay. The NBT assay is simple, sensitive, quantitative, and can be used to determine the amounts of intracellular superoxide anion radicals (O2•-) produced by a wide variety of cells.16,17
KYSE-70 cells or HEEpiCs, at a density of 2.0 × 104, were seeded in a 35 mm dish and incubated for 24 hours at 37°C in 95% humidified air and 5% CO2. The cell culture media were replaced with 2 mL of H2-silica-containing media, and the cells were incubated for 3 days. The media were then replaced with 300 μL of 0.2% NBT (Boehringer Ingelheim, Rheinland-Pfalz, Germany) solution, which was dissolved in distilled water. After 1.5 hours, the NBT solution was aspirated, and the reaction was stopped by the addition of 500 μL of prechilled PBS (–). The cells were fixed in 70% methanol at -20°C for 1 minute. The unreduced NBT dye was completely removed by washing the wells twice with 2 mL of cold PBS (–). Then, 1 mL of cold PBS (–) was added to each well and photomicrographs of stained cells were prepared using a camera (Meiji Techno Co., Ltd., MT5310H, Tokyo, Japan). The supernatant was aspirated, and the formazan formed from NBT was dissolved in 1 mL of a 1:1 v/v mixture of 4 M potassium hydroxide (KOH):dimethyl sulfoxide (DMSO) in each well. The plate was set on an orbital shaker for 30 minutes, and the nitroblue formazan in the extract was determined at 680 nm with a spectrophotometer (V-630 iRM, Nihon Bunko Corp., Tokyo, Japan).18
Western blot assay for detection of activated Caspase-3 and Bax/Bcl-2
KYSE-70 cells or HEEpiCs treated with H2-silica were seeded at approximately 1 × 106 cells per T-25 flask (BD Biosciences) and were further cultured for 3 days. The cells were collected with a small rubber scraper (TR9000, TrueLine, Nashville, TN, USA), centrifuged at 5,000 r/min at 4°C for 5 minutes, and then washed twice in ice-cold PBS. After that, the cell pellet was homogenized in 60 μL of cold lysis buffer (150 mM NaCl, 10 mM Tris–HCl (pH 7.4), 1% Triton X-100, 1% NP-40, 5 mM EDTA (pH 8.0), and 1/200 vol. of Protease Inhibitor Cocktail Set III (Calbiochem, CA, USA, #539134). Following centrifugation at 14,000 r/min at 4°C for 5 minutes, 7 μL of the supernatant was mixed with 1/2 vol. of 4× NuPAGE LDS sample buffer (Invitrogen) and 1/4 vol. of 10× NuPAGE antioxidant (Invitrogen), and run on a 13.5% acrylamide sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel.
Additional procedures were the same as reported in our previous study,19 except that anti-Bax (BioLegend, CA, USA, #625901), anti-Bcl-2 (eBioscience, CA, USA, #141028), and anti-a-tubulin (BioLegend, #643801) were utilized as the primary antibodies and peroxidase-conjugated affipure goat anti-mouse IgG (H+L)* (#115-035-003) was utilized as the second antibody. Finally, the ratio of the intensity of activated caspase-3 versus that of α-tubulin and Bax versus Bcl-2 were used to evaluate the occurrence of apoptosis in KYSE-70 cells or HEEpiCs.20
Statistical analysis
Data are presented as the mean ± standard deviation, and compared with the Student’s t-test using Microsoft Excel 2013 or SPSS 11.0 (SPSS Inc., Chicago, IL, USA) for Windows. A P value of < 0.05 was considered to indicate statistical significance.
RESULTS
Cell viability
In Figure 2, the summarized results are derived from experiments using WST-8 staining to measure the cell proliferation and viability of both cell lines. These indicate that H2-silica could decrease the proliferation and viability of KYSE-70 cells at concentrations of 300, 600, and 1,200 ppm (P < 0.05 or P < 0.01). Meanwhile, H2-silica could promote the proliferation and viability of HEEpiCs at 300 and 600 ppm (P < 0.01).
Figure 2.
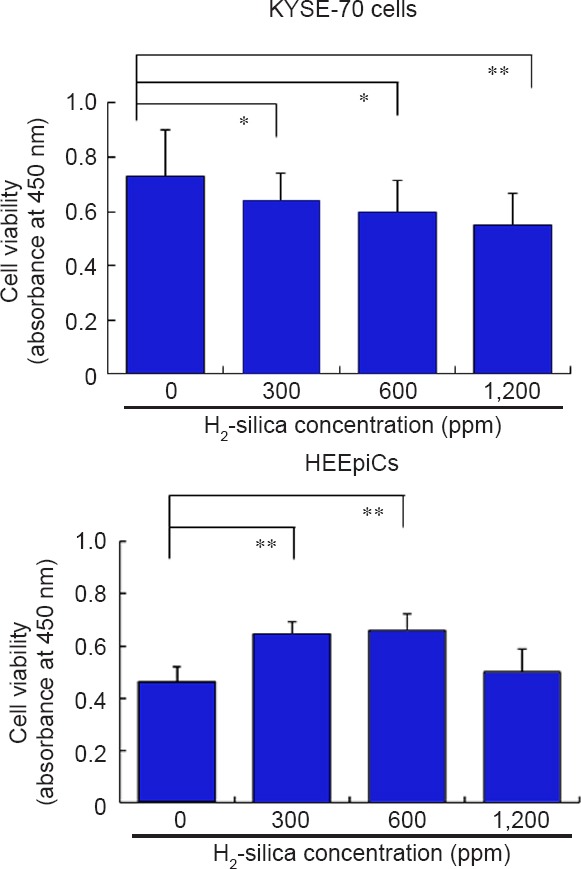
Cell viability of KYSE-70 cells and HEEpiCs treated with H2-silica detected by WST-8 staining.
Note: Data are expressed as the mean ± standard deviation. The 0 ppm group was used as a control. Analyses were done using Student's t-test. *P < 0.05, **P < 0.01. WST: 4-[3-(2-methoxy-4-nitro-phenyl)-2-[4-nitrophenyl]-2H-5-tetrazolio]-1,3-benzene disulfonate sodium salt; HEEpiCs: human esophageal epithelial cells; H2-silica: hydrogen-occluding-silica.
Effect of H2-silica on the migration of KYSE-70 cells and HEEpiCs
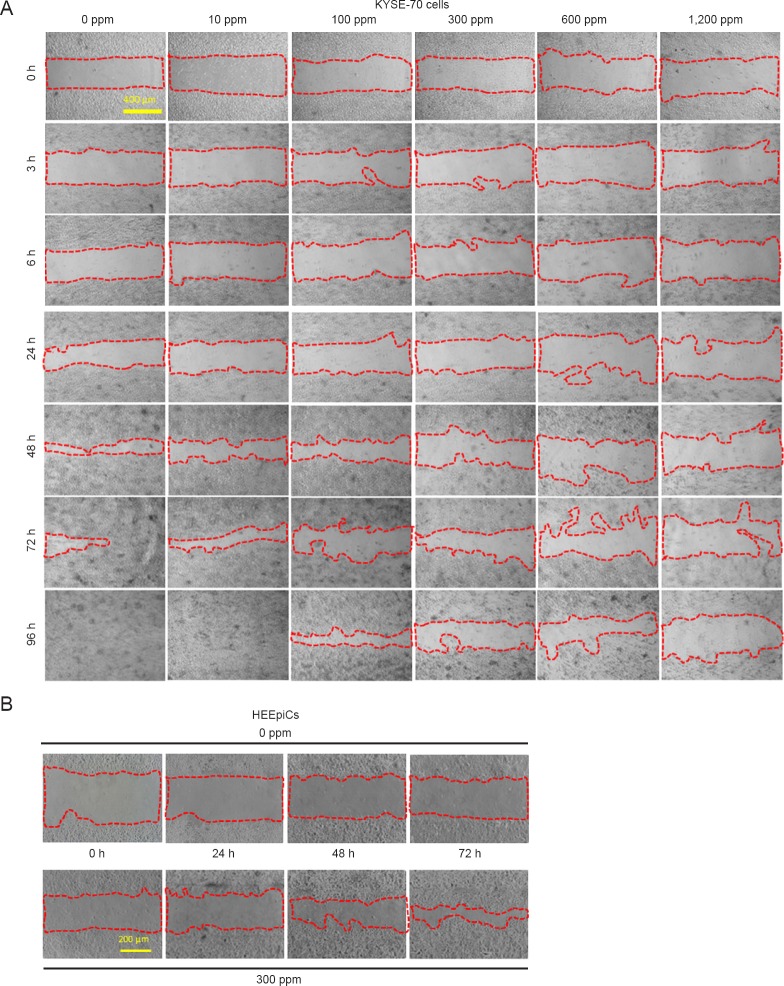
A wound-healing assay was performed to observe how the morphology of KYSE-70 cells changed during migration. There were differences in gap closure in each H2-silica treated group compared with the control (0 ppm). There were significant changes beyond 48 hours, particularly at 72 and 96 hours, At 96 hours in the 0 and 10 ppm groups, the gaps were completely closed (Figure 3A). This shows that gap closure depended on different concentrations of H2-silica. These results indicate that H2-silica can inhibit the cell migration of KYSE-70 cells. In contrast, as shown in Figure 3B, 300 ppm H2-silica had a facilitatory effect on the migration of HEEpiCs. However, 600 and 1,200 ppm did not promote migration (data not shown).
Figure 3.
Cell migration is reduced by H2-silica administration in KYSE-70 and HEEpiCs (× 40).
Note: (A) Phase contrast images of the wound-healing assay in KYSE-70 cells at 0, 3, 6, 24, 48, 72, and 96 h after wound scratching. (B) Phase contrast images of HEEpiCs at 0, 24, 48, and 72 h after chamber removal in a culture-insert migration assay. Bars: 400 μm in A, 200 μm in B. HEEpiCs: Human esophageal epithelial cells; h: hour(s); H2-silica: hydrogen-occluding-silica.
Intracellular ROS levels in KYSE-70 cells and HEEpiCs
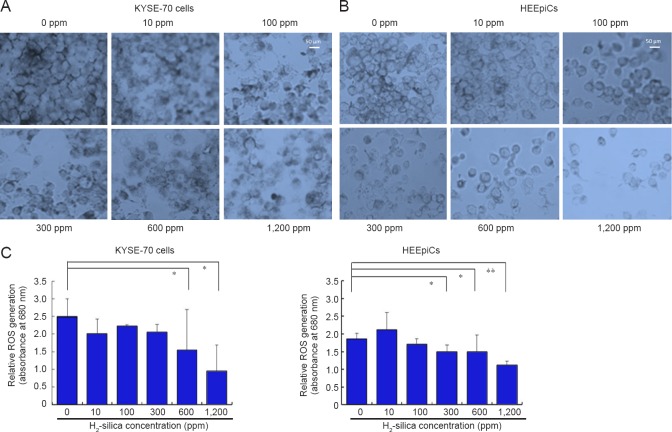
The NBT assay was used to evaluate intracellular levels of ROS (superoxide radicals) in KYSE-70 cells and HEEpiCs (Figure 4). Intracellular levels of superoxide radical tended to decrease with increasing H2-silica concentrations. The morphologic changes observed in KYSE-70 cells 2 days after H2-silica administration are shown in Figure 4A. Quantification of superoxide radical levels is presented in Figure 4C (left). The increase in the added amount of H2-silica enabled confirmation that the ROS activity in whole cells decreased. In contrast, the morphologic changes observed in HEEpiCs 4 days after H2-silica treatment are shown in Figure 4B. Evaluation based on relative ROS generation rate was shown in Figure 4C (right). An H2-silica-concentration-dependent cell change was observed concurrently with lowered cell densities. These results indicated that generation of superoxide radicals was not related to treatment with H2-silica.
Figure 4.
Photomicrographs of KYSE-70 cells and HEEpiCs: NBT assay.
Note: (A) Visualization of NBT staining in KYSE-70 cells (× 400). (B) Visualization of NBT staining in HEEpiCs (× 400). Bars: 50 μm. (C) Quantitative results of intracellular ROS detected by NBT staining in KYSE-70 cells (left) and HEEpiCs (right). There was a tendency for intracellular levels of ROS to decrease with increasing H2-silica concentrations. Data are expressed as the mean ± standard deviation. Student's t-test was used for the statistical analysis. *P < 0.05, **P < 0.01. HEEpiCs: Human esophageal epithelial cells; ROS: reactive oxygen species; NBT: nitroblue tetrazolium.
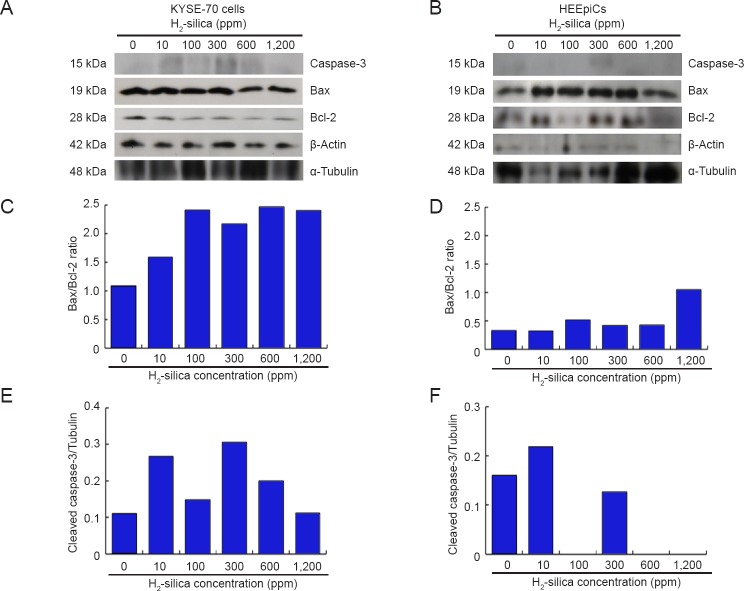
Activated Caspase-3 and Bax/Bcl-2 expressions in KYSE-70 cells and HEEpiCs
Western blot assay was performed to investigate activated (cleaved) caspase-3 and Bax/Bcl-2 expressions that are thought to be the markers for mammalian cell apoptotic pathways. In fact, the Bcl-2 family includes some regulator proteins that regulate cell death (apoptosis), either by inducing (proapoptotic) or inhibiting (antiapoptotic) apoptosis.18 The elevated levels of 15 kDa bands that corresponded to processed and activated caspase-313 were expressed in KYSE-70 cells at 10, 300 and 600 ppm, or in HEEpiCs at only at 10 ppm (Figure 5A, B). The 19 kDa bands corresponding to processed Bax were expressed in KYSE-70 cells and HEEpiCs (Figure 5A, B). The tendency was for a gradual increase in the observed Bax/Bcl-2 ratio (Figure 5C, D) in KYSE-70 cells, particularly beyond 10 or 100 ppm concentrations of H2-silica. On the other hand, HEEpiCs did not show a noticeable increase at H2-silica concentrations of 10, 100, 300, or 600 ppm; however, at 1,200 ppm, the cells had a remarkable increase in Bax/Bcl-2 ratio compared with those at 0 ppm (control). Above all, these results suggested that H2-silica possesses an apoptosis-inducing effect.
Figure 5.
Western blot analysis of activated caspase-3 and Bax/Bcl-2 ratio in KYSE-70 cells and HEEpiCs.
Note: (A, B) The immuno-detected bands of activated caspase-3 (15 kDa), Bax (19 kDa), Bcl-2 (28 kDa), β-actin (42 kDa) and α-tubulin (48 kDa) at different concentrations of H2-silica in both cell lines. (C, D) The ratios of band densities for Bax/Bcl-2 of KYSE-70 cells and HEEpiCs, respectively. (E, F) The ratios (%) of band densities for activated caspase-3 versus α-tubulin and for Bax/Bcl-2 of KYSE-70 cells and HEEpiCs, respectively. HEEpiCs: Human esophageal epithelial cells; H2-silica: hydrogen-occluding-silica.
DISCUSSION
In this study, we used several assays and methods to inspect whether H2-silica produced a carcinostatic effect on KYSE-70 cells in vitro. Using the WST-8 assay, we found an inhibitory effect on KYSE-70 cell proliferation within 48 hours after H2-silica administration. The respective inhibitory effects on cell migration were also shown through the wound-healing assay, those reflect invasion and metastasis of cancer cells.21 In addition, from the results of the ratios of activated caspase-3 and Bax/Bcl-2 experiment, it was confirmed that H2-silica had the effect of inducing and enhancing cancer cell apoptosis.
In order to observe and compare the other adverse effects that occur in the surrounding normal cells during anticancer treatment, we also obtained experimental data from HEEpiCs. Although there was an inhibitory effect on KYSE-70 cells, only a high concentration (1,200 ppm) of H2-silica led to prominent apoptosis in HEEpiCs. These results suggest that H2-silica has an inhibitory effect on human cancer cells, but that only high concentrations of H2-silica result in cytotoxic effects on normal human cells. We previously observed that the autoclaved and dehydrogenated H2-silica scarcely showed the antioxidant and cytoprotective effects, assumedly through the mechanical inhibition due to contact between silica microparticles-cells, for human keratinocytes HaCaT, which, furthermore, suffered from a slight inhibition for cell proliferation above the definite dose of H2-silica which was seemed to be considerably different from one cell line to another cell line.12 On the other hand, we cannot exclude a possibility for the specific toxicity from silica itself or by the pH change of the medium due to addition of silica.
Much research has reported on hydrogen gas or hydrogen water since the breakthrough paper of Ohsawa et al.3 in 2007. However, studies in which cancer is the experimental target have been scarce.22 We noted with interest that the first paper about the possible biological effects of hydrogen gas on human skin cancer cells was reported in 1975.23 Although this paper did not report positive results, the fact that the focus was on cancer cells is admirable. Recently, hydrogen water plus some kind of antioxidant was investigated in spite of rare papers that reported inhibitory effects on cancer cells with hydrogen water alone in vitro.
Saitoh et al.24 reported that platinum-nanocolloid-supplemented hydrogen water could inhibit either colony formation efficiencies or colony sizes of human tongue carcinoma cells. Nakashima-Kamimura et al.25 observed that inhalation of hydrogen gas improved mortality and body-weight loss caused by cisplatin, and alleviated nephrotoxicity in vitro and in vivo. However, hydrogen did not impair the antitumor activity of cisplatin against cancer cell lines in vitro or in vivo in tumor-bearing mice. They concluded that hydrogen has the potential to improve the quality of life of patients during chemotherapy by efficiently mitigating the side effects of cisplatin.25 Asada et al.26 also reported that hydrogen water in combination with platinum nanocolloid decreased cell proliferation, shrinkage, pyknosis, and karyorrhexis of Ehrlich ascites tumor cells. Moreover, Runtuwene et al.27 investigated the anticancer effect of hydrogen water in combination with 5-fluorouracil. They found that hydrogen water administration enhanced cell apoptosis, resulting in a marked increase in the expression of phosphorylated AMP-activated protein kinase, apoptosis-inducing factor, and caspase-3 in colon 26 cells. Additionally, high-concentration hydrogen water exhibited stronger antioxidative and anticancer activities than did low-concentration hydrogen water.
The KYSE-70 cells that were used in this study were malignant squamous epithelial cells derived from human esophageal carcinoma tissue.28 KYSE-70 cells belong to stage IIB, T1 or T2 (T1: tumor invades lamina propria or submucosa; T2: tumor invades muscularis propria) in the American Joint Committee on Cancer (AJCC) staging systems.29,30 Hence, we chose KYSE70 cells as our experimentally cultured cancer cell line.13 In the present study, we observed the cell migration of KYSE-70 cells.
Cell migration plays a central role in a wide variety of biological phenomena.31,32 As shown in Figure 2, H2-silica has a certain antiproliferative effect on KYSE-70 cells, particularly during the initial 48 hours after treatment (P < 0.05). In addition, the inhibitory effect of H2-silica on cancer cell migration lasted beyond 48 hours. Compared with the control at 72 hours, a clear difference was not observed. Furthermore, inhibition of filopodia formation and closure of a scratch wound were observed in comparison with the control group. By microscopic observation, it was seen that H2-silica inhibited hypertrophy and other cellular processes of cancer cells.
The H2-silica that was used in the present study is a hydride-based compound with H-ions interstitially embedded in a matrix of caged silica. Synthesis is simple and efficient with consistent results of about 17% w/w hydride content. A paper reported the ORP, pH, relative hydrogen score, and 1H-nuclear magnetic resonance (NMR) spectra for H2-silica, and indicated that it had a significant reduction potential of -750 mV.33 However, silica is a health-beneficial, biologically friendly compound with the capacity to scavenge and reduce ROS. Silica in the state of silicon, as well as fine silicon dioxide, is used in food additives and cosmetics and is currently approved for use by The Ministry of Health, Labour and Welfare in Japan (http://www.ffcr.or.jp/zaidan/MHWinfo.nsf). It should be emphasized that the H2-silica used for this experiment is a different substance to crystalline silica, which belongs to group 1 as defined by the International Agency for Research on Cancer (IARC).34 By definition, group 1 materials can be carcinogenic to humans through dust inhalation, especially for the erionite that is a fibrous component of some natural zeolite deposits.
Kato et al.12 investigated the anti-melanogenic efficacy of H2-silica in human melanin-generating pigment cells (HMV-II) that were subjected to oxidative stress by ultraviolet A (UVA) light exposure. After UVA irradiation, HMV-II cells were stimulated to produce melanin 2.72-fold more abundantly than the untreated control cells. When HMV-II cells were treated with H2-silica at a low concentration of 20 ppm pre- and post-UVA irradiation, the amount of melanin was repressed to 12.20% compared with that of the control cells. The investigators found that cell viability and apoptosis events were unchanged even at high-level concentrations of 100–1,000 ppm. Our results are consistent with those from the report by Kato et al.12
According to our results showing that H2-silica can inhibit proliferation, promote apoptosis, and prevent cell migration in KYSE-70 cells, H2-silica may be a potential therapeutic agent in cancer prevention and inhibition of metastasis through its anti-ROS effects.
Piskounova et al.35 reported that oxidative stress could limit distant metastasis in vivo. This could explain the phenomenon in our experiment that the migration of KYSE-70 cells showed a remarkable inhibitory tendency depending on an increased concentration of H2-silica (Figure 3A).
Malignant cells are often maintained in a high metabolic state, so their ROS levels are higher than those of normal cells. In order to maintain this rapid growth state, malignant cells must keep growth factor pathways activated. This can cause tumor cells to absorb more nutrients, and enhance cell division signals. Hence, this state will inevitably lead to mitochondria, endoplasmic reticulum, and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase producing more and more ROS. Scientists have found that ROS promote cancer mainly through signal conditioning rather than through the destruction of chromosome structure.
The results obtained from our experiments seem to indicate that potent antioxidants can cause sensitization if they are used on tumor cells that maintain high levels of ROS. In contrast, H2-silica may produce toxic effects in normal cells that maintain low levels of ROS.
As shown in Figure 3C, H2-silica at a low concentration has a gentle promotive effect on the migration of HEEpiCs. This suggests that many growth factor signaling pathways act through phosphorylation to begin with and end through dephosphorylation, and maintain a state of being ready to start by stopping dephosphorylation. ROS play an important role in the regulation of phosphorylation and dephosphorylation. First, growth factors such as platelet-derived growth factor and epithelial growth factor can quickly enhance the generation of ROS, mainly through the NADPH oxidase pathway. Second, ROS are important for the autophosphorylation of growth factor-induced tyrosine residues.
H2-silica exhibited anticancer activity in KYSE-70 cells by affecting antioxidant enzymes, possibly leading to H2O2 accumulation, cell cycle arrest at the G2/M phase, and apoptotic cell death via the death receptor and mitochondrial apoptotic pathways.
After H2-silica administration, differences were seen between the two cell types in the Bax/Bcl-2 ratio. H2-silica administration in the cancer cells showed an inhibitory effect and induced apoptosis at a high concentration (1,200 ppm). However, there were no obvious changes in cell viability or apoptotic events even at concentrations of 100, 300, or 600 ppm. In contrast, the normal cells responded differently to H2-silica. It was found that administering H2-silica at low or moderate concentrations (≤ 300 ppm) did not damage normal cells.
Administration to normal cells at a high concentration (1,200 ppm) caused a decrease in the viability of the cells, an increase in induction of apoptosis, and cytotoxicity. It is well known that induction of unwanted apoptosis is harmful to the growth and proliferation of normal cells. A too powerful antioxidant may be good to eliminate the cancer cells, but it will produce an adverse consequence such as a cytotoxic effect on the surrounding normal cells through mechanisms such as cell apoptosis or necrosis.
The results obtained from the present study suggested that administration of H2-silica to either KYSE-70 cells would promote cell apoptosis; however for HEEpiCs the effect was not so drastic. In view of the increased Bax/Bcl-2 ratios after H2-silica administration, it is possible that apoptosis had been induced in cancer cell lines selectively especially in the pathway of Bax and Bcl-2.36,37 It suggests that H2-silica may sometimes trigger cell death via the non-caspase pathway or the initiator-caspase-skipping route rather than the typical effector caspase-3 mediated pathway, or it is possible that activated caspase-3 would be degraded by other proteases. This result suggests the same mechanism as revealed by our previous studies.13,19
We believe that there are several limitations to this study that warrant further research. The first is the negative results from the NBT assay. Ohsawa et al.3 found that the inhalation of H2 gas selectively reduced hydroxyl radicals, and concluded that H2 may effectively protect cells in vitro or in vivo. In general, the hydroxyl radical is thought to be the most cytotoxic of the ROS. The reasons for the negative results in our study were clear, but we will focus on different methods of administrating H2 and intend to use another ROS detection method in the future.
We also intend to obtain some data to explain the relationship between the cytoskeleton and cell apoptosis in future research. In addition, we were not able to not confirm whether H2-silica 300 ppm produces a proliferative effect in HEEpiCs between 0 and 96 hours, or after 96 hours (Figure 3C). We will therefore analyze the time course and other concentrations of H2-silica than 300 ppm to perform a similar study on human fibroblasts.
Finally, it is clear that H2-silica particle is not only portable and easy to store but also safer than H2 generating apparatus which is expensive, difficult to manipulate, and has the potential to explode. H2-silica delivers hydrogen gas in a certain way; therefore, it will be used in our future studies for observing wound repair in human normal skin cells in vitro.
Footnotes
Funding: This work was supported in part by a Grant-in-Aid for Scientific Research (KAKENHI No. 26350681, Qiang Li) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflicts of interest
The authors declare no conflict of interest.
Contributor agreement
A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check
This paper has been checked twice with duplication-checking software iThenticate.
Peer review
A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Open peer reviewer
Xue-jun Sun, Second Military Medical University, China.
REFERENCES
- 1.Sun X OS, Nakao A. Hydrogen Molecular Biology and Medicine. Springer; 2015. [Google Scholar]
- 2.Huang CS, Kawamura T, Toyoda Y, Nakao A. Recent advances in hydrogen research as a therapeutic medical gas. Free Radic Res. 2010;44:971–982. doi: 10.3109/10715762.2010.500328. [DOI] [PubMed] [Google Scholar]
- 3.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 4.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 5.Sun Q, Kawamura T, Masutani K, et al. Oral intake of hydrogen-rich water inhibits intimal hyperplasia in arterialized vein grafts in rats. Cardiovasc Res. 2012;94:144–153. doi: 10.1093/cvr/cvs024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noda K, Tanaka Y, Shigemura N, et al. Hydrogen-supplemented drinking water protects cardiac allografts from inflammation-associated deterioration. Transpl Int. 2012;25:1213–1222. doi: 10.1111/j.1432-2277.2012.01542.x. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda K, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun. 2007;361:670–674. doi: 10.1016/j.bbrc.2007.07.088. [DOI] [PubMed] [Google Scholar]
- 8.Shi Q, Chen C, Deng WH, et al. Hydrogen-rich saline attenuates acute hepatic injury in acute necrotizing pancreatitis by inhibiting inflammation and apoptosis, involving JNK and p38 mitogen-activated protein kinase-dependent reactive oxygen species. Pancreas. 2016;45:1424–1431. doi: 10.1097/MPA.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 9.Stephanson CJ, Stephanson AM, Flanagan GP. Antioxidant capability and efficacy of Mega-H silica hydride, an antioxidant dietary supplement, by in vitro cellular analysis using photosensitization and fluorescence detection. J Med Food. 2002;5:9–16. doi: 10.1089/109662002753723179. [DOI] [PubMed] [Google Scholar]
- 10.Stephanson CJ, Flanagan GP. Antioxidant capacity of silica hydride: a combinational photosensitization and fluorescence detection assay. Free Radic Biol Med. 2003;35:1129–1137. doi: 10.1016/s0891-5849(03)00495-7. [DOI] [PubMed] [Google Scholar]
- 11.Stephanson CJ, Flanagan GP. Differential metabolic effects on mitochondria by silica hydride using capillary electrophoresis. J Med Food. 2004;7:79–83. doi: 10.1089/109662004322984743. [DOI] [PubMed] [Google Scholar]
- 12.Kato S, Saitoh Y, Miwa N. Inhibitions by hydrogen-occluding silica microcluster to melanogenesis in human pigment cells and tyrosinase reaction. J Nanosci Nanotechnol. 2013;13:52–59. doi: 10.1166/jnn.2013.6848. [DOI] [PubMed] [Google Scholar]
- 13.Li Q, Tanaka Y, Saitoh Y, Tanaka H, Miwa N. Carcinostatic effects of platinum nanocolloid combined with gamma irradiation on human esophageal squamous cell carcinoma. Life Sci. 2015;127:106–114. doi: 10.1016/j.lfs.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 14.Pinco KA, He W, Yang JT. Alpha4beta1 integrin regulates lamellipodia protrusion via a focal complex/focal adhesion-independent mechanism. Mol Biol Cell. 2002;13:3203–3217. doi: 10.1091/mbc.02-05-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer N, Walzl A, Unger C, et al. In vitro cell migration and invasion assays. Mutat Res. 2013;752:10–24. doi: 10.1016/j.mrrev.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- 17.Choi HS, Kim JW, Cha YN, Kim C. A quantitative nitroblue tetrazolium assay for determining intracellular superoxide anion production in phagocytic cells. J Immunoassay Immunochem. 2006;27:31–44. doi: 10.1080/15321810500403722. [DOI] [PubMed] [Google Scholar]
- 18.Kato S, Saitoh Y, Iwai K, Miwa N. Hydrogen-rich electrolyzed warm water represses wrinkle formation against UVA ray together with type-I collagen production and oxidative-stress diminishment in fibroblasts and cell-injury prevention in keratinocytes. J Photochem Photobiol B. 2012;106:24–33. doi: 10.1016/j.jphotobiol.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Li Q, Tanaka Y, Saitoh Y, Miwa N. Effects of platinum nano-colloid in combination with gamma irradiation on normal human esophageal epithelial cells. J Nanosci Nanotechnol. 2016;16:5345–5352. doi: 10.1166/jnn.2016.12362. [DOI] [PubMed] [Google Scholar]
- 20.Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642–652. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohta S. Molecular hydrogen as a novel antioxidant: overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015;555:289–317. doi: 10.1016/bs.mie.2014.11.038. [DOI] [PubMed] [Google Scholar]
- 23.Dole M, Wilson FR, Fife WP. Hyperbaric hydrogen therapy: a possible treatment for cancer. Science. 1975;190:152–154. doi: 10.1126/science.1166304. [DOI] [PubMed] [Google Scholar]
- 24.Saitoh Y, Okayasu H, Xiao L, Harata Y, Miwa N. Neutral pH hydrogen-enriched electrolyzed water achieves tumor-preferential clonal growth inhibition over normal cells and tumor invasion inhibition concurrently with intracellular oxidant repression. Oncol Res. 2008;17:247–255. doi: 10.3727/096504008786991620. [DOI] [PubMed] [Google Scholar]
- 25.Nakashima-Kamimura N, Mori T, Ohsawa I, Asoh S, Ohta S. Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemother Pharmacol. 2009;64:753–761. doi: 10.1007/s00280-008-0924-2. [DOI] [PubMed] [Google Scholar]
- 26.Asada R, Kageyama K, Tanaka H, et al. Antitumor effects of nano-bubble hydrogen-dissolved water are enhanced by coexistent platinum colloid and the combined hyperthermia with apoptosis-like cell death. Oncol Rep. 2010;24:1463–1470. doi: 10.3892/or_00001006. [DOI] [PubMed] [Google Scholar]
- 27.Runtuwene J, Amitani H, Amitani M, Asakawa A, Cheng KC, Inui A. Hydrogen-water enhances 5-fluorouracil-induced inhibition of colon cancer. Peer J. 2015;3:e859. doi: 10.7717/peerj.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimada Y, Imamura M, Wagata T, Yamaguchi N, Tobe T. Characterization of 21 newly established esophageal cancer cell lines. Cancer. 1992;69:277–284. doi: 10.1002/1097-0142(19920115)69:2<277::aid-cncr2820690202>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 29.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 30.Kim HI, Cheong JH, Song KJ, et al. Staging of adenocarcinoma of the esophagogastric junction: comparison of AJCC 6th and 7th gastric and 7th esophageal staging systems. Ann Surg Oncol. 2013;20:2713–2720. doi: 10.1245/s10434-013-2898-5. [DOI] [PubMed] [Google Scholar]
- 31.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 32.Trepat X, Chen Z, Jacobson K. Cell migration. Compr Physiol. 2012;2:2369–2392. doi: 10.1002/cphy.c110012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stephanson CJ, Flanagan GP. Synthesis of a novel anionichydride organosiloxane presenting biochemical properties. Inter J Hydrogen Energy. 2003;28:1243–1250. [Google Scholar]
- 34.Wilbourn JD, McGregor DB, Partensky C, Rice JM. IARC reevaluates silica and related substances. Environ Health Per-sped. 1997;105:756–759. doi: 10.1289/ehp.97105756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piskounova E, Agathocleous M, Murphy MM, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–191. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller TM, Moulder KL, Knudson CM, et al. Bax deletion further orders the cell death pathway in cerebellar granule cells and suggests a caspase-independent pathway to cell death. J Cell Biol. 1997;139:205–217. doi: 10.1083/jcb.139.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun ZG, Chen LP, Wang FW, Xu CY, Geng M. Protective effects of ginsenoside Rg1 against hydrogen peroxide-induced injury in human neuroblastoma cells. Neural Regen Res. 2016;11:1159–1164. doi: 10.4103/1673-5374.187057. [DOI] [PMC free article] [PubMed] [Google Scholar]