Abstract
Assessment of tumor response after chemotherapy using 18F-FDG PET metrics is gaining acceptance. Several studies have suggested that the parameters metabolically active tumor volume (MTV) and total lesion glycolysis (TLG) are superior to SUVmax for measuring tumor burden. However, the measurement of MTV and TLG is still controversial; the most common method uses an absolute threshold of 42% of SUVmax. Recently, we implemented a background-adaptive method to determine the background-subtracted lesion activity (BSL) and the background-subtracted volume (BSV). In this study, we investigated the correlation between such PET metrics and histopathologic response in non–small cell lung carcinoma (NSCLC).
Methods
Forty-four NSCLC patients were retrospectively identified. Their PET/CT data on both types of scan before and after neoadjuvant chemotherapy were analyzed regarding SUVmax, MTV, TLG, BSL, and BSV, as well as the relative changes in these parameters. The tumor regression score as an indicator of histopathologic response was scored on hematoxylin- and eosin-stained sections of the surgical specimens using a 4-tiered scale (scores 1–4). The correlation between score and the absolute and relative PET metrics after chemotherapy was analyzed using Spearman rank correlation tests.
Results
Tumors that demonstrated a good response after neoadjuvant chemotherapy had significantly lower 18F-FDG activity than non-responding tumors (scores 3 and 4: SUVmax, 4.2 [range, 1.8–7.9] vs. scores 1 and 2: SUVmax, 8.1 [range, 1.4–40.4]; P = 0.001). The same was found for change in SUVmax and score (P = 0.001). PET volume metrics based on a 42% fixed threshold for SUVmax did not correlate with score (TLG, P = 0.505; MTV, P = 0.386). However, both background activity–based PET volume metrics—BSL and BSV—significantly correlated with score (P < 0.001 each).
Conclusion
PET volume metrics based on background-adaptive methods correlate better with histopathologic score in NSCLC patients under neoadjuvant chemotherapy than algorithms and methods using a fixed threshold (42% SUVmax).
Keywords: volume segmentation, tumor regression, quantification, NSCLC, neoadjuvant therapy
Assessment of response to therapy is a quickly growing field for 18F-FDG PET/CT in oncology imaging (1). The correlation between SUVmax and histopathologic tumor regression on radiotherapy (2) or neoadjuvant chemotherapy (3) has been established in non–small cell lung cancer (NSCLC) patients. However, some limiting factors, such as increased macrophage infiltration, may result in a falsely elevated SUVmax (4), and false-negative results may occur for large, bulky lesions in which residual vital tumor is present despite a complete metabolic response according to SUVmax (5). Nevertheless, a complete metabolic response on 18F-FDG PET is superior to CT volume assessment for evaluating histopathologic response (6). de Geus-Oei et al. analyzed the metabolic rate of glucose and the SUV after induction chemotherapy and showed that a decrease in SUVmean of more than 35% correlated best with a favorable progression-free and overall survival (7).
Recent publications focusing on 18F-FDG PET segmentation to assess total tumor burden, including metabolically active tumor volume (MTV) and total lesion glycolysis (TLG), showed a superior correlation with progression-free and overall survival of NSCLC compared with SUVmax (8–11). In particular, a multivariate analysis identified TLG and treatment method (surgery vs. other) to be the only two independent prognostic factors for progression-free survival (12). TLG and MTV were suggested by Larson et al. in 1999 to determine total tumor burden and volume using PET data (13). With the combination of CT and PET images, volume measurement via PET became less central for NSCLC, because tumor volume can easily be assessed on CT. However, with an increasing number of publications suggesting that MTV and TLG are superior in assessing NSCLC response compared with SUVmax, the question of how to measure them is relevant again (14,15).
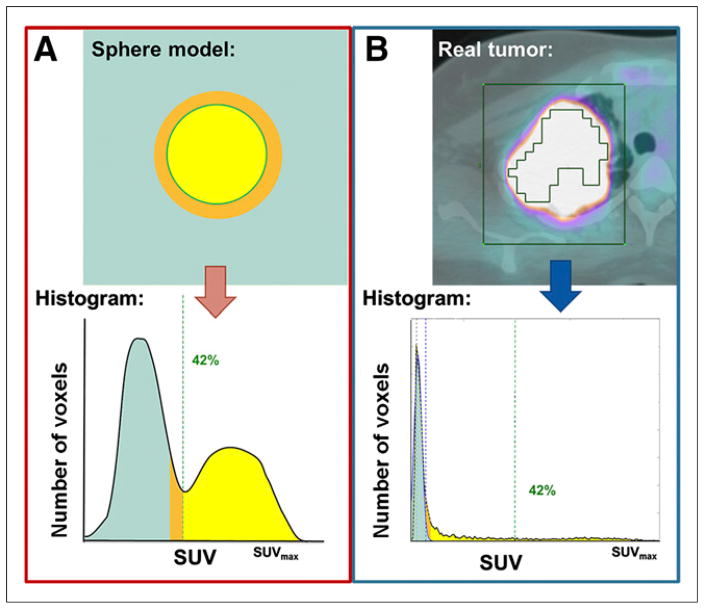
In most of the recent studies, MTV and TLG were computed using a fixed SUVmax threshold of 40%–50% (16–18). We have recently shown that this threshold method has limitations for the measurement of lesion activity and volume: MTV and TLG based on a fixed threshold using SUVmax (e.g., 42%) will underestimate lesional uptake with a high activity and overestimate lesions with an SUVmax close to the background level (19). Other authors have used a fixed SUV threshold, most commonly 2.5 (20–22), with the obvious limitations of an arbitrary cutoff. Lesions with low activity are thereby underestimated or not even measurable. We have therefore developed a background-based estimation including background-subtracted lesion activity (BSL) and background-subtracted volume (BSV) (Fig. 1). Our method uses a single volume of interest (VOI) that surrounds the tumor and reads its histogram to measure the total activity within the lesion (19). It is based on our previously published study showing that background activity can be assessed automatically within the tumor VOI using histogram analysis (23). Because background voxels have more homogeneous values, they will always represent the mode in a selected VOI, with a normal gaussian distribution. Therefore, background voxels can be removed from the VOI by subtraction of a normal distribution fitted to the first peak in the histogram (19).
FIGURE 1.
The two segmentation methods. (A) Use of an absolute SUVmax threshold of 42% to delineate the ideal homogeneous sphere has been shown to optimally segment the true volume of the sphere (yellow). (B) For heterogeneous real tumors, however, a 42% cutoff would miss a substantial number of tumor voxels (yellow), whereas subtraction of the gaussian normal distribution representing background (green) includes all voxels with activity above background, yielding BSV.
In this study, we used 18F-FDG PET to test the performance of the relative change in several tumor volume metrics—MTV, TLG, BSV, and BSL—for NSCLC after treatment with neoadjuvant chemotherapy. As an independent reference for tumor response, we chose the histopathologic tumor regression score of the corresponding formalin-fixed and paraffin-embedded surgical specimens, as described by Junker et al. (24,25).
MATERIALS AND METHODS
Patient Selection
Patients with locally advanced stage II, stage III, or oligometastatic stage IV disease underwent neoadjuvant chemotherapy according to international guidelines following the decision of our local tumor board (26). To be selected for our study, between January 2002 and December 2012 the patient must have undergone 18F-FDG PET/CT before receiving neoadjuvant treatment and before undergoing surgery. This retrospective study was approved by the Ethical Commission of the Canton of Zurich, and the requirement to obtain informed consent was waived.
From a total of 92 NSCLC patients undergoing surgery after neoadjuvant chemotherapy, 44 met all inclusion criteria. Most patients were stage III at diagnosis. After neoadjuvant treatment, only 3 patients showed tumors with ypT0, whereas ypT3 predominated. Demographic details are given in Table 1.
TABLE 1.
Characteristics of the 44 Patients
| Characteristic | Data* | % |
|---|---|---|
| Demographics | ||
|
| ||
| Median age at surgery (y) | 62 (range, 38–75) | |
| Median body weight (kg) | 70 (range, 43–123) | |
| Median18F-FDG dose (MBq) | 352 (range, 274–430) | |
| Sex (male/female) | 25/19 | 56.8/43.2 |
|
| ||
| Histology | ||
|
| ||
| Squamous cell carcinoma | 23 | 52.3 |
| Adenocarcinoma | 21 | 47.7 |
|
| ||
| Clinical stage at diagnosis | ||
|
| ||
| II | 6 | 13.6 |
| III | 35 | 79.5 |
| IV | 3 | 6.8 |
|
| ||
| Tumor location | ||
|
| ||
| Left lower lobe | 6 | 13.6 |
| Left upper lobe | 15 | 34.1 |
| Right lower lobe | 6 | 13.6 |
| Right middle lobe | 3 | 6.8 |
| Right upper lobe | 14 | 31.8 |
|
| ||
| Pathologic stage after chemotherapy | ||
|
| ||
| 0 | 3 | 6.8 |
| I | 5 | 11.4 |
| II | 10 | 22.7 |
| III | 25 | 56.8 |
| IV | 1 | 2.3 |
|
| ||
| CT-based clinical T-stage at diagnosis | ||
|
| ||
| cT1 | 3 | 6.8 |
| cT2 | 25 | 56.8 |
| cT3 | 9 | 20.5 |
| cT4 | 7 | 15.9 |
|
| ||
| Pathologic T-stage after chemotherapy | ||
|
| ||
| ypT0 | 3 | 6.8 |
| ypT1 | 5 | 11.4 |
| ypT2 | 10 | 22.7 |
| ypT3 | 25 | 56.8 |
| ypT4 | 1 | 2.3 |
|
| ||
| Chemotherapy | ||
|
| ||
| Median cycles | 3 (range, 2–6) | |
| Platinum/gemcitabine | 17 | 51.6 |
| Platinum/taxane | 25 | 56.8 |
| Other | 3 | 6.8 |
Data are n, except where otherwise indicated.
PET/CT Acquisition and Analysis
The inclusion criteria for 18F-FDG PET/CT were as follows: scans of adequate quality, a fasting period of at least 4 h, no elevation in blood glucose, an 18F-FDG uptake time of 45–60 min, and an adequate 18F-FDG injection (<100-MBq difference between the two 18F-FDG injections).
All patients were examined using a routine clinical protocol in the Institute of Nuclear Medicine on dedicated PET/CT scanners (DSTX [GE Healthcare], 16- or 64-slice CT, 7–8 frames, frame time of 1.5 or 2 min) with injection of 350 MBq of 18F-FDG 45–60 min before examination. A low-dose unenhanced CT scan was performed for attenuation correction and used for anatomic localization (80 mA, 140 kV). The imaging findings were analyzed by a physician dually board-certified in nuclear medicine and radiology, who was masked to the histopathology results.
A VOI was placed around the primary tumor in such a way that the entire tumor activity was enclosed and regions of physiologically increased activity were avoided (e.g., 18F-FDG uptake by the heart). If high-activity structures could not be avoided, they were cut out before the analysis. Instructions on VOI placement were previously published (23). In brief, the VOI had to be slightly larger than the tumor. For lesions with a heterogeneous background (e.g., tumors abutting lung and mediastinal tissue or hilar vessels), the VOIs were adjusted to include more of the background tissue with higher 18F-FDG activity (e.g., mediastinum). Within the selected VOI, SUVmax, MTV, TLG, BSL, and BSV were measured. The change in these 5 PET metrics before and after neoadjuvant chemotherapy was also calculated. On CT, the maximal tumor diameter was measured in 3 dimensions (a, b, and c) and tumor volume, CTvol, was estimated as an ellipsoid using the formula 4/3π(a/2 × b/2 × c/2), along with the corresponding change in CTvol (27).
Histopathologic Assessment of Tumor Regression
For histopathologic assessment, the inclusion criteria were the availability of at least 2 representative original whole-tumor hematoxylin- and eosin-stained slides for regression scoring, no secondary simultaneous tumor, and a histologic subtype of either adenocarcinoma or squamous cell carcinoma. Only the primary tumors were analyzed. All hematoxylin- and eosin-stained resection specimens processed for the original sign-out were reviewed by two of the authors to determine the score, which was based on a 4-tiered scale as described by Junker et al. (24,25). This system evaluates the proportion of viable tumor cells in relation to the degree of tumor necrosis and fibrosis. In brief, score 1 is defined as no tumor regression or only minor, mostly spontaneous, regression; score 2 is defined as the presence of more than 10% vital tumor tissue; score 3 is defined as less than 10% vital tumor epithelia in all tumors; and score 4 is defined as the presence of complete tumor regression whereby only fibrotic and necrotic areas with macrophage-rich xanthomatous inflammation remain in the original tumor volume.
For dichotomized data analysis, scores of 1 and 2 were regarded as indicating low regression and therefore the tumors nonresponding, whereas tumors with scores 3 or 4 were considered responders.
Statistical Analysis
The distribution of changes in PET metrics for the various regression scores was analyzed using box plots. Correlations between scores and the absolute and relative PET metrics, as well as CTvol, were calculated using Spearman rank tests. A receiver-operator-characteristic curve was generated for SUVmax after chemotherapy and for all 5 PET metrics for score-based responders versus nonresponders. The area under the curve (AUC) was calculated. The optimal cutoff for the receiver-operator-characteristic curve was determined by applying the Youden index to each cutoff value (maximum of sensitivity + specificity − 1) (28).
RESULTS
Thirteen patients had a score or 3 or 4 and thus were considered responders. Only 1 patient did not show any histologic regression (score 1), whereas 30 patients had a score of 2 on histopathology. Lesions with a good response to neoadjuvant chemotherapy (score 3 or 4) had a mean SUVmax of 4.2 (range, 1.8–7.9), whereas lesions regarded as nonresponders (score 1 or 2) had a mean SUVmax of 8.1 (range, 1.4–40.4). An SUVmax cutoff of less than 6.4 after neoadjuvant chemotherapy yielded a sensitivity and specificity of 85% and 58%, respectively, for predicting a good pathologic response.
Differences between responders and nonresponders were significant for change in SUVmax (P = 0.001), BSL, and BSV (P < 0.001, respectively), whereas change in TLG and MTV was not significantly different between responders and nonresponders (Table 2).
TABLE 2.
Absolute Values for PET Metrics after Chemotherapy in Nonresponders vs. Responders
| PET metric after chemotherapy | Nonresponder (score 1 or 2, n = 31)
|
Responder (score 3 or 4, n = 13)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Median | Min | Max | Mean | Median | Min | Max | |
| SUVmax | 8.1 | 7.3 | 1.4 | 40.4 | 4.2 | 4.0 | 1.8 | 7.9 |
|
| ||||||||
| TLG | 59.3 | 38.7 | 6.8 | 229.9 | 41.1 | 45.0 | 9.8 | 76.4 |
|
| ||||||||
| MTV | 13.9 | 9.1 | 1.1 | 43.8 | 23.2 | 15.1 | 5.9 | 69.1 |
|
| ||||||||
| BSL | 82.2 | 32.3 | 0.2 | 344.6 | 22.4 | 9.5 | 1.1 | 76.6 |
|
| ||||||||
| BSV | 19.5 | 9.6 | 0.1 | 82.2 | 6.7 | 3.1 | 0.7 | 20.6 |
Min = minimum; max = maximum.
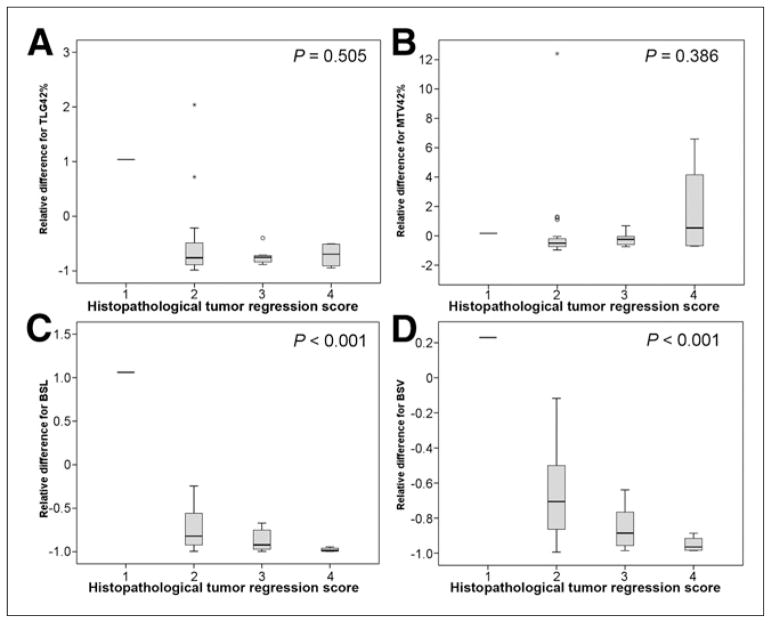
Using the Spearman rank test, the correlation between change in SUVmax and score had a P value of 0.001. PET volume metrics based on a fixed SUVmax threshold did not correlate with score (P = 0.505 for change in TLG and 0.386 for change in MTV). However, both of the background activity–based PET volume metrics correlated significantly with score (P < 0.001 for both BSL and BSV) (Fig. 2).
FIGURE 2.
Box plots showing no correlation between score and change in PET volume metrics based on fixed SUVmax threshold of 42% (TLG [A] and MTV [B]) but significant correlation between score and background-based metrics (BSL [C] and BSV [D]).
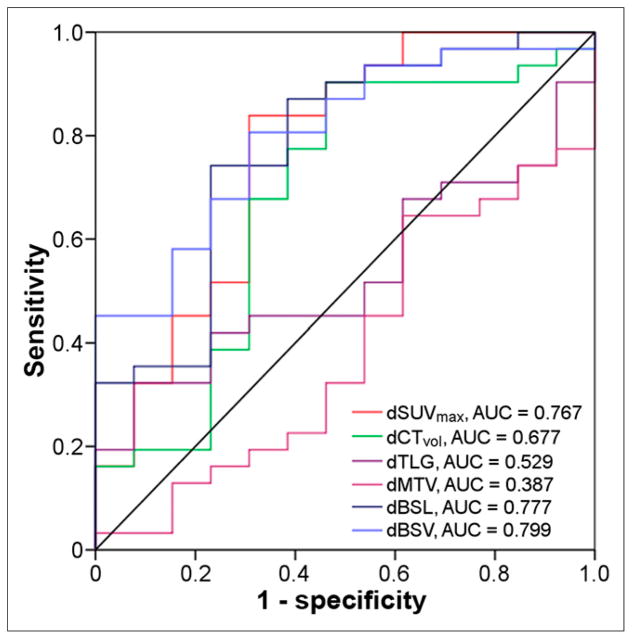
Receiver-operator-characteristic analysis showed the largest AUC to be for BSV (0.799), followed by BSL (0.777) and SUVmax (0.767), whereas TLG and MTV had AUCs of 0.529 and 0.387, respectively (Fig. 3). A −68% cutoff for change in SUVmax showed a sensitivity and specificity of 69% and 84%, respectively. For change in BSV, a −88% cutoff gave a sensitivity and specificity of 69% and 81%, respectively, and for change in BSL, a −90% cutoff gave a sensitivity and specificity of 77% and 74%, respectively (Table 3).
FIGURE 3.
Receiver-operator-characteristic curve for change in SUVmax, MTV, TLG, BSL, and BSV and scores 1–2 vs. 3–4. BSV had the largest AUC (0.799), with a −88% cutoff yielding sensitivity and specificity of 69% and 81%, respectively. SUVmax and BSL were not significantly inferior, with AUCs of 0.767 and 0.777, respectively. Prefix d denotes relative change.
TABLE 3.
Receiver-Operator-Characteristic Curve Analysis, with Corresponding Cutoffs, Sensitivities, and Specificities
| PET metric | Area | SE | Asymptotic significance | Asymptotic 95% CI | Cutoff | Sensitivity | Specificity |
|---|---|---|---|---|---|---|---|
| SUVmax II | 0.759 | 0.073 | 0.007 | 0.615–0.903 | 6.4 | 85% | 58% |
| Change in… | |||||||
| CT volume | 0.677 | 0.097 | 0.066 | 0.488–0.867 | −92% | 54% | 90% |
| SUVmax | 0.767 | 0.086 | 0.006 | 0.598–0.936 | −68% | 69% | 81% |
| TLG | 0.529 | 0.087 | 0.767 | 0.357–0.700 | NA | NA | NA |
| MTV | 0.387 | 0.092 | 0.242 | 0.206–0.568 | NA | NA | NA |
| BSL | 0.777 | 0.080 | 0.004 | 0.620–0.934 | −90% | 74% | 77% |
| BSV | 0.799 | 0.069 | 0.002 | 0.663–0.935 | −88% | 69% | 84% |
CI = confidence interval; NA = not applicable.
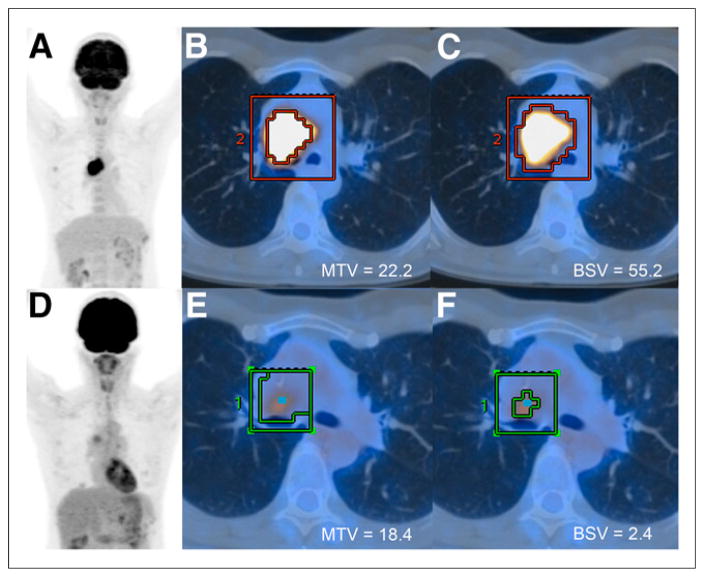
Figure 4 illustrates a case of stage IIIA central adenocarcinoma in the right hilum with high 18F-FDG activity (SUVmax, 16.1) before neoadjuvant chemotherapy and a good partial metabolic response after the chemotherapy (SUVmax, 4.9 [−70%]). However, MTV decreased only minimally, from 22.2 to 18.4 cm3 (−17%), whereas BSV decreased substantially, from 55.2 to 2.4 cm3 (−96%). Histopathologic examination showed extensive fibroelastotic scar tissue with small residual foci of vital adenocarcinoma growing in lepidic fashion along the alveolar walls, corresponding to a score of 3 (Fig. 5).
FIGURE 4.
52-y-old woman treated with cisplatin/pemetrexed-based neoadjuvant chemotherapy for stage IIIA central adenocarcinoma of right hilum (SUVmax, 16.1). (A–C) Staging 18F-FDG PET/CT image with coronal maximum-intensity projection overview (A) and axial fused PET/CT images with tumor VOI around lesion (B and C), illustrating that MTV gives significantly lower volume than BSV in lesion with high activity. (D–F) Restaging 18F-FDG PET/CT study after 3 cycles of chemotherapy, showing significant decrease in SUVmax to 4.9, correlating with score of 3. Although BSV decreased by 96%, MTV decreased by only 17%, not entirely reflecting a good tumor response.
FIGURE 5.
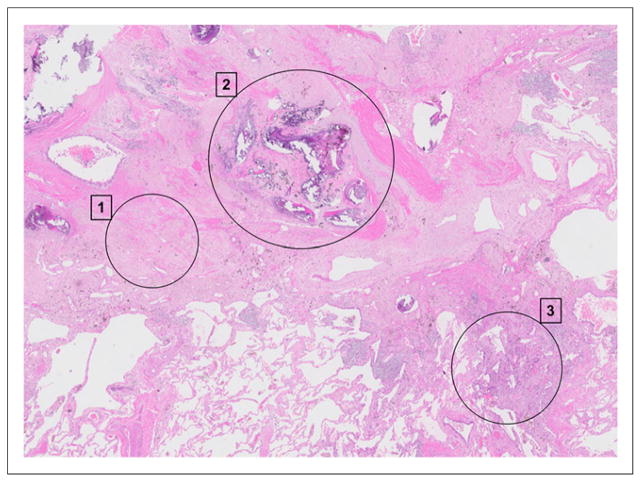
Corresponding histologic whole section for patient in Figure 4, with score of 3. (1) Extensive fibroelastotic scar tissue after neoadjuvant chemotherapy (partial response), with normal, partially emphysematic lung seen at bottom left. (2) Central portion of tumor, consisting of large calcifications, heavy elastosis, complete vessel remodeling, and enclosed anthracotic pigment. (3) Small residual focus of vital adenocarcinoma growing in lepidic fashion along alveolar walls. (Hematoxylin and eosin, ×10.)
DISCUSSION
Our results showed that SUVmax after neoadjuvant chemotherapy could distinguish responders from nonresponders with a high sensitivity of 85%, although the specificity, at 58%, was rather low. Change in SUVmax was associated with tumor regression, and a −68% cutoff predicted good to complete tumor regression with a specificity of 81% (scores 3 and 4). This finding is in line with previous studies that suggested a decrease in SUVmax to be associated with good tumor response (29–31). One study investigated the relation between quantitative 18F-FDG metrics and pathologic tumor response and showed a linear relation between change in SUVmax and percentage of nonviable tumor (32). Those investigators also came to the conclusion that metabolic parameters were superior to CT morphology for response assessment. This conclusion agrees with our results showing that a decrease in change in CTvol yielded an AUC of 0.677, compared with 0.767 for SUVmax and 0.799 for BSL.
An increasing number of studies are using an SUV of 2.5 as an absolute threshold, especially for segmentation of lung tumors (20–22). In our cohort, one adenocarcinoma had an SUVmax of 2.4 before chemotherapy, which, for a threshold of 2.5, would not have been measurable with TLG or MTV. Moreover, this adenocarcinoma showed a decrease of only 50% and 60% in BSL and BSV, respectively, suggesting only a partial metabolic response, which was confirmed by histopathology (score 2). Furthermore, 8 lesions had an SUVmax of less than 2.5 after chemotherapy but only 4 were complete responders; of the 4 nonresponding lesions, BSL and BSV suggested complete response for only one.
Previously published papers suggest that PET volume metrics such as MTV and TLG are superior to SUVmax for predicting overall and progression-free survival. However, MTV and TLG based on a fixed threshold (SUVmax, 42%) failed to predict histopathologic response in the current study, whereas the background-adapted segmentation methods correlated with score. This might be explained by phantom results showing that a fixed threshold can substantially underestimate TLG and MTV for lesions with high 18F-FDG uptake (19). A decrease in SUVmax during therapy therefore may falsely increase MTV because a larger volume of less active tumor will be included in the MTV. This possibility can be illustrated by 18F-FDG PET/CT examinations before and after neoadjuvant chemotherapy as shown in Figure 4, in which MTV overestimated the volume after therapy whereas BSV showed a good response. Histopathologic examination confirmed tumor regression with a score of 3 (Fig. 5).
The original paper by Erdi et al. also suggested that PET tumor segmentation requires an adapted threshold based on the tumor-to-background ratio (33). Drawing a separate VOI over the background for every lesion is time-consuming, especially in patients with multiple metastases. Therefore, we suggest our histogram-based 1-step method of measuring tumor activity and estimating volume. For tumor volume definition before radiotherapy planning, however, Nestle et al. (34) have already suggested background-based tumor segmentation with a separate VOI drawn over the background area. Other investigators have looked at histogram indices such as SD, skewness, kurtosis, entropy, and energy, but the results are controversial and those metrics were not part of the current study (35).
In the present study, change in SUVmax was not significantly inferior to change in BSL or BSV for predicting tumor regression. This result may reflect the importance of finding the most aggressive part within a tumor as reflected by the highest SUV, as compared with MTV, and needs to be investigated in larger cohorts.
The study had several limitations. The retrospective nature of the analysis led to some inconsistencies in uptake time, and the injected 18F-FDG dose varied over time. Care was taken to exclude patients with high blood sugar, paravenous injection, or scan artifacts (motion, metal implants). In addition, the cohort represented a real patient population within the selected clinical setting, and quantification measures should be reliable in such a setting, too. Also we did not perform outcome analysis; we investigated the direct correlation between PET quantification and histopathology and believe that this patient population is too small and heterogeneous (stage, therapy, histology) to allow a meaningful assessment of correlation with progression-free and overall survival. Therefore, follow-up projects on larger homogeneous patient cohorts that underwent primary surgery without neoadjuvant chemotherapy are planned.
BSL and BSV are not intended to serve as a PET segmentation tool, since space information is lost in the histogram and therefore selected voxels do not correspond one-to-one to the voxels in the images. BSV therefore may not provide the accurate segmentation of the tumor boundary that is necessary for radiation therapy planning. Subtraction of background activity from the tumor VOI will show the BSL to be the entire amount of activity coming from the tumor regardless of its location; that is, spill-out and spill-over will be included. As a result, for delineation of tumor volume, a spill-over correction is necessary but was not performed in this study because we were interested in the change in total activity/volume and not the boundary. The 42% SUVmax threshold suggested by Erdi can be applied only for homogeneous spheres with high lesion-to-background ratios (33). The heterogeneous nature of real tumors might be better reflected by Nestle’s suggestion of taking 15% of the average activity plus background to delineate the tumor area for radiotherapy (34). In assessing tumor burden through imaging, the fact that spillover is regarded as a part of tumor volume might only weakly affect overall accuracy, as suggested by the fact that the correlation to score was stronger for BSV and BLS than for MTV and TLG.
CONCLUSION
The current data confirm that PET volume metrics based on a fixed SUVmax threshold (42%) lead to a significant bias and do not correlate with response to chemotherapy as assessed by histopathologic examination. PET volume metrics based on background-adapted measurements, however, correlate with tumor regression.
Footnotes
DISCLOSURE
Irene A. Burger was financially supported by the Prof. Dr. Max Cloëtta Foundation (Switzerland) and the Swiss Society of Nuclear Medicine. Ruben Casanova is supported by the Swiss Cancer League (F-87701-31-01). This research was funded in part through NIH/NCI Cancer Center Support Grant P30 CA008748. No other potential conflict of interest relevant to this article was reported.
References
- 1.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mac Manus MP, Hicks RJ, Matthews JP, et al. Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2003;21:1285–1292. doi: 10.1200/JCO.2003.07.054. [DOI] [PubMed] [Google Scholar]
- 3.Nahmias C, Hanna WT, Wahl LM, Long MJ, Hubner KF, Townsend DW. Time course of early response to chemotherapy in non-small cell lung cancer patients with 18F-FDG PET/CT. J Nucl Med. 2007;48:744–751. doi: 10.2967/jnumed.106.038513. [DOI] [PubMed] [Google Scholar]
- 4.Poettgen C, Theegarten D, Eberhardt W, et al. Correlation of PET/CT findings and histopathology after neoadjuvant therapy in non-small cell lung cancer. Oncology. 2007;73:316–323. doi: 10.1159/000134474. [DOI] [PubMed] [Google Scholar]
- 5.Ryu JS, Choi NC, Fischman AJ, Lynch TJ, Mathisen DJ. FDG-PET in staging and restaging non-small cell lung cancer after neoadjuvant chemoradiotherapy: correlation with histopathology. Lung Cancer. 2002;35:179–187. doi: 10.1016/s0169-5002(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 6.Schmücking M, Baum RP, Bonnet R, Junker K, Muller KM. Pathologe. 2005;26:178–189. doi: 10.1007/s00292-005-0758-1. [DOI] [PubMed] [Google Scholar]
- 7.de Geus-Oei LF, van der Heijden HF, Visser EP, et al. Chemotherapy response evaluation with 18F-FDG PET in patients with non-small cell lung cancer. J Nucl Med. 2007;48:1592–1598. doi: 10.2967/jnumed.107.043414. [DOI] [PubMed] [Google Scholar]
- 8.Chung HW, Lee KY, Kim HJ, Kim WS, So Y. FDG PET/CT metabolic tumor volume and total lesion glycolysis predict prognosis in patients with advanced lung adenocarcinoma. J Cancer Res Clin Oncol. 2014;140:89–98. doi: 10.1007/s00432-013-1545-7. [DOI] [PubMed] [Google Scholar]
- 9.Melloni G, Gajate AM, Sestini S, et al. New positron emission tomography derived parameters as predictive factors for recurrence in resected stage I non-small cell lung cancer. Eur J Surg Oncol. 2013;39:1254–1261. doi: 10.1016/j.ejso.2013.07.092. [DOI] [PubMed] [Google Scholar]
- 10.Zaizen Y, Azuma K, Kurata S, et al. Prognostic significance of total lesion glycolysis in patients with advanced non-small cell lung cancer receiving chemotherapy. Eur J Radiol. 2012;81:4179–4184. doi: 10.1016/j.ejrad.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Wroblewski K, Liao S, et al. Prognostic value of metabolic tumor burden from 18F-FDG PET in surgical patients with non-small-cell lung cancer. Acad Radiol. 2013;20:32–40. doi: 10.1016/j.acra.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Chen HH, Chiu NT, Su WC, Guo HR, Lee BF. Prognostic value of whole-body total lesion glycolysis at pretreatment FDG PET/CT in non-small cell lung cancer. Radiology. 2012;264:559–566. doi: 10.1148/radiol.12111148. [DOI] [PubMed] [Google Scholar]
- 13.Larson SM, Erdi Y, Akhurst T, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. the visual response score and the change in total lesion glycolysis. Clin Positron Imaging. 1999;2:159–171. doi: 10.1016/s1095-0397(99)00016-3. [DOI] [PubMed] [Google Scholar]
- 14.Hyun SH, Ahn HK, Kim H, et al. Volume-based assessment by 18F-FDG PET/CT predicts survival in patients with stage III non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2014;41:50–58. doi: 10.1007/s00259-013-2530-8. [DOI] [PubMed] [Google Scholar]
- 15.Usmanij EA, de Geus-Oei LF, Troost EG, et al. 18F-FDG PET early response evaluation of locally advanced non-small cell lung cancer treated with concomitant chemoradiotherapy. J Nucl Med. 2013;54:1528–1534. doi: 10.2967/jnumed.112.116921. [DOI] [PubMed] [Google Scholar]
- 16.Chang KP, Tsang NM, Liao CT, et al. Prognostic significance of 18F-FDG PET parameters and plasma Epstein-Barr virus DNA load in patients with nasopharyngeal carcinoma. J Nucl Med. 2012;53:21–28. doi: 10.2967/jnumed.111.090696. [DOI] [PubMed] [Google Scholar]
- 17.Vargas HA, Burger IA, Goldman DA, et al. Volume-based quantitative FDG PET/CT metrics and their association with optimal debulking and progression-free survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery. Eur Radiol. 2015;25:3348–3353. doi: 10.1007/s00330-015-3729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallicchio R, Mansueto G, Simeon V, et al. F-18 FDG PET/CT quantization parameters as predictors of outcome in patients with diffuse large B-cell lymphoma. Eur J Haematol. 2014;92:382–389. doi: 10.1111/ejh.12268. [DOI] [PubMed] [Google Scholar]
- 19.Burger IA, Vargas HA, Apte A, et al. PET quantification with a histogram derived total activity metric: superior quantitative consistency compared to total lesion glycolysis with absolute or relative SUV thresholds in phantoms and lung cancer patients. Nucl Med Biol. 2014;41:410–418. doi: 10.1016/j.nucmedbio.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JW, Kang CM, Choi HJ, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with pancreatic cancer. J Nucl Med. 2014;55:898–904. doi: 10.2967/jnumed.113.131847. [DOI] [PubMed] [Google Scholar]
- 21.Chung HH, Kim JW, Han KH, et al. Prognostic value of metabolic tumor volume measured by FDG-PET/CT in patients with cervical cancer. Gynecol Oncol. 2011;120:270–274. doi: 10.1016/j.ygyno.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Seol YM, Kwon BR, Song MK, et al. Measurement of tumor volume by PET to evaluate prognosis in patients with head and neck cancer treated by chemo-radiation therapy. Acta Oncol. 2010;49:201–208. doi: 10.3109/02841860903440270. [DOI] [PubMed] [Google Scholar]
- 23.Burger IA, Vargas HA, Beattie BJ, et al. How to assess background activity: introducing a histogram-based analysis as a first step for accurate one-step PET quantification. Nucl Med Commun. 2014;35:316–324. doi: 10.1097/MNM.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 24.Junker K, Langner K, Klinke F, Bosse U, Thomas M. Grading of tumor regression in non-small cell lung cancer: morphology and prognosis. Chest. 2001;120:1584–1591. doi: 10.1378/chest.120.5.1584. [DOI] [PubMed] [Google Scholar]
- 25.Junker K, Thomas M, Schulmann K, Klinke F, Bosse U, Muller KM. Tumour regression in non-small-cell lung cancer following neoadjuvant therapy: histological assessment. J Cancer Res Clin Oncol. 1997;123:469–477. doi: 10.1007/BF01192200. [DOI] [PubMed] [Google Scholar]
- 26.Vansteenkiste J, De Ruysscher D, Eberhardt WEE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013l;24(suppl 6):vi89–vi98. doi: 10.1093/annonc/mdt241. [DOI] [PubMed] [Google Scholar]
- 27.Formula for geometrical figures. [Accessed March 4, 2016];Israel Science and Technology website. http://www.science.co.il/Formula.asp.
- 28.Fluss R, Faraggi D, Reiser B. Estimation of the Youden index and its associated cutoff point. Biom J. 2005;47:458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 29.Burger IA, Schwarz EI, Samarin A, Breitenstein S, Weber A, Hany TF. Correlation between therapy response assessment using FDG PET/CT and histopathologic tumor regression grade in hepatic metastasis of colorectal carcinoma after neoadjuvant therapy. Ann Nucl Med. 2013;27:177–183. doi: 10.1007/s12149-012-0670-8. [DOI] [PubMed] [Google Scholar]
- 30.Capirci C, Rampin L, Erba PA, et al. Sequential FDG-PET/CT reliably predicts response of locally advanced rectal cancer to neo-adjuvant chemo-radiation therapy. Eur J Nucl Med Mol Imaging. 2007;34:1583–1593. doi: 10.1007/s00259-007-0426-1. [DOI] [PubMed] [Google Scholar]
- 31.Palma P, Conde-Muino R, Rodriguez-Fernandez A, et al. The value of metabolic imaging to predict tumour response after chemoradiation in locally advanced rectal cancer. Radiat Oncol. 2010;5:119. doi: 10.1186/1748-717X-5-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerfolio RJ, Bryant AS, Winokur TS, Ohja B, Bartolucci AA. Repeat FDG-PET after neoadjuvant therapy is a predictor of pathologic response in patients with non-small cell lung cancer. Ann Thorac Surg. 2004;78:1903–1909. doi: 10.1016/j.athoracsur.2004.06.102. [DOI] [PubMed] [Google Scholar]
- 33.Erdi YE, Mawlawi O, Larson SM, et al. Segmentation of lung lesion volume by adaptive positron emission tomography image thresholding. Cancer. 1997;80:2505–2509. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2505::aid-cncr24>3.3.co;2-b. [DOI] [PubMed] [Google Scholar]
- 34.Nestle U, Kremp S, Schaefer-Schuler A, et al. Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-small cell lung cancer. J Nucl Med. 2005;46:1342–1348. [PubMed] [Google Scholar]
- 35.Orlhac F, Soussan M, Maisonobe JA, Garcia CA, Vanderlinden B, Buvat I. Tumor texture analysis in 18F-FDG PET: relationships between texture parameters, histogram indices, standardized uptake values, metabolic volumes, and total lesion glycolysis. J Nucl Med. 2014;55:414–422. doi: 10.2967/jnumed.113.129858. [DOI] [PubMed] [Google Scholar]