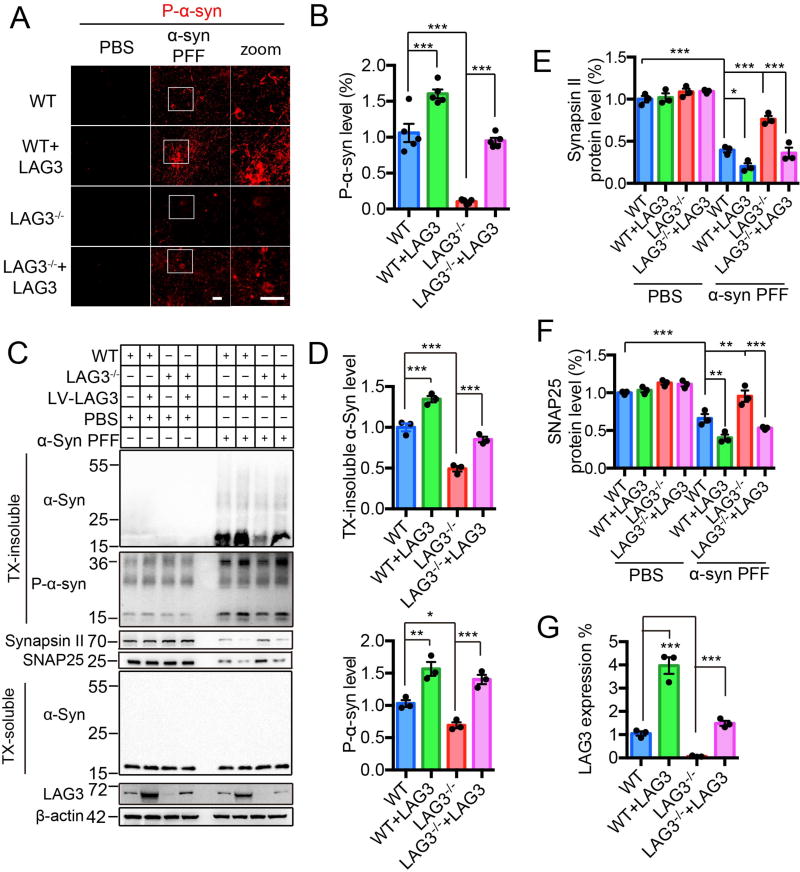
Fig. 3. α-Syn PFF induced pathology is reduced by deletion of LAG3 in vitro.
(A) WT and LAG3−/− primary cortical neurons at 7 DIV were treated with α-syn PFF or PBS. LAG3 was overexpressed via Lenti-virus (LV) transduction in WT or LAG3−/− neurons at 4 DIV. 3 days after transduction, 7 DIV cultures were treated with α-syn PFF or PBS. All the cultures were fixed 10-day post-treatment in 4% PFA. Neurons were stained with rabbit mAb MJF-R13 (8-8) for P-α-syn. Scale bar, 40 µm. (B) Quantification of panel A, n = 5 independent experiments, each performed in duplicate. Values are given as the means ± SEM. Statistical significance was determined using one-way ANOVA followed with Tukey’s correction, ***P < 0.001. Power (1-β err prob) = 1. (C) Immunoblots in WT and LAG3−/− neuron lysates of misfolded α-syn, P-α-syn, synapsin II, SNAP25 and LAG3. β-actin served as a loading control. WT and LAG3−/− neuron lysates were sequentially extracted in 1% TX-100 (TX-soluble) followed by 2% SDS (TX-insoluble) 14 days after α-syn PFF treatment. α-Syn PFF recruited endogenous α-Syn into TX-insoluble and hyperphosphorylated aggregates, which was ameliorated by deletion of LAG3. α-Syn PFF caused a reduction in levels of SNAP25 and synapsin II compared to PBS 14 days post-treatment. Deletion of LAG3 prevented PFF-induced synaptic protein loss. (D–G) Quantification of panel C. Values are given as means ± SEM, n = 3 independent experiments. Statistical significance was determine using one-way ANOVA followed by Tukey’s correction, *P < 0.05, **P < 0.01, ***P < 0.001.