Abstract
Inclusion body hepatitis and hepatitis–hydropericardium syndrome caused by high-pathogenic fowl adenovirus serotype 4 has recently plagued Chinese poultry industry and caused huge economic losses since 2013. So far, there is no commercial vaccine available to control this disease. In this study, we reported the development of both embryo-adapted and cell-culture derived inactivated FAdV-4 vaccines and evaluated their efficacies in chicken. Compared to embryo-adapted vaccine, cell-culture derived vaccine induced significantly earlier and higher serological response measured by AGP and ELISA. After virus challenge, chicken immunized with cell-culture derived vaccine did not showed any gross and histopathological lesions, whereas inclusion body hepatitis was observed in the liver of chicken vaccinated with embryo-adapted vaccine. No mortality was observed in both the vaccinated groups. The above results suggested that cell-culture derived FAdV-4 inactivated vaccine could be a better vaccine candidate than embryo-adapted vaccine to control FADV-4 infections in China.
Keywords: Fowl adenovirus serotype 4, Inactivated vaccine, Hydropericardium–hepatitis syndrome (HHS), Inclusion body hepatitis (IBH), LMH cells
Introduction
Inclusion body hepatitis (IBH) and hydropericardium–hepatitis syndrome (HHS) are infectious diseases affecting poultry especially broiler birds [11, 19]. The unique features of HHS are accumulation of transparent or straw-colored fluid in the pericardial sac, and hepatitis with basophilic intra-nuclear inclusion bodies [1, 26, 27]. The IBH and HHS are caused by fowl adenovirus (FAdV) which belongs to the genus Aviadenovirus within the family Adenoviridae.
Adenoviruses are non-enveloped, icosahedral particle measuring 70–100 nm in diameter, with 12 vertices and 20 triangular faces composed of 252 capsomers [2]. The avian adenoviruses have been grouped into five species (FAdV-A to FAdV-E) based on their molecular structure and further divided into 12 serotypes (FAdV-1 to 8a and 8b to 11) by their serological relationships [17]. According to previous studies, pathogenesis of FAdV infection is different within the serotypes or genotypes [14, 15]. Although all 12 serotypes of FAdVs were reported to be associated with outbreaks of IBH [14, 28], FAdV serotype 4 and 11 (FAdV-4 and 11) can also cause HHS [16, 31].
Since 2015, many outbreaks of HHS characterized by chicken pericardial effusion and hepatitis were reported from China leading to huge economic losses in China [20]. FAdV-4 has been confirmed to be the causative agent for HHS [20, 30].
To control the disease, FAdV subunit vaccines based on penton base protein or capsid protein were expressed in Escherichia coli and baculovirus system, respectively [23, 25]. Unfortunately, these subunit vaccines failed to provide fully protection against challenge with virulent FAdV-4. In 2011, Mansoor et al. [16] reported that a chicken embryo-adapted autologous FAdV-4 vaccine made from local strain of virus could provide good protection to the homologous challenge. In 2014, a cell-culture derived FAdV-4 inactivated vaccine was reported to provide broad cross-protection against various serotypes of fowl adenovirus [12]. Unfortunately, there was no FAdV-4 commercial vaccine available to control the disease in China. In this study, we report the development of both embryo-adapted and cell-culture derived inactivated FAdV-4 vaccines and their efficacies against challenge with a homologous virulent FAdV-4 isolate from Henan province in China.
Materials and methods
Viruses and vaccine preparation
FAdV-4 HN strain (FAV-HN) was isolated from liver of infected birds which showed clinical signs of hydropericardium–hepatitis syndrome (HHS) in Henan province, China as previously described [16]. Serotype of the FAdV-4 isolate was defined by phylogenetic analysis based on the nucleotide sequences of Hexon gene according to ICTV system [3]. This isolate was used for virus propagation in chicken embryo and cell culture, vaccine preparation and a challenge study.
To adapt the virus on chicken embryos, 7-day-old specific pathogen-free (SPF) chicken embryos (Merial, France) was used to inoculate virus via yolk sac route. Allantoic fluid was harvested and filtered through 0.2 µm membrane filters (Millipore, USA) each time for consecutive 4 passages. Allantoic fluid of 4th passage virus was collected and inactivated with formaldehyde (0.03% in final product). The inactivation of virus was confirmed by three blind passages in LMH cells with no cytopathic effect. Chicken embryo-adapted vaccine was prepared by emulsifying the above antigen solution with light mineral oils (MARCOL 52, France) at a ratio of 33:67 (V/V). The final dose of the inactivated embryo-adapted vaccine contains 1 × 106 median tissue culture infective dose (TCID50) per bird in 0.3 mL.
To prepare cell-culture derived FAdV-4 inactivated vaccine, FAV-HN strain propagation was performed in chicken hepatocellular carcinoma cell line (LMH, ATCC) cultured in Waymouth’s MB 752/1 medium supplemented with 10% fetal bovine serum (FBS). Confluent cell monolayers and virus-inoculated cultures were maintained in Waymouth’s MB 752/1 medium with 2% FBS. The virus titer was determined in 96-well microtitre plates as described previously [4]. Formaldehyde (0.03% in final product) was added for the inactivation of above virus. The inactivation of virus was confirmed as above mentioned. The cell-culture derived vaccine was prepared by emulsifying the formaldehyde-inactivated FAdV-4 antigen solution with light mineral oils at a ratio of 33:67 (V/V). The final dose of the inactivated oil-emulsion inactivated FAdV-4 vaccine was 1 × 106 TCID50 per bird in 0.3 mL.
Antibody response of vaccinated chicken
To evaluate antibody response of inactivated FAdV-4 vaccine, forty 21-day old SPF chicken were randomly divided into four groups with ten chickens in each group. Chicken in group A was immunized subcutaneously with 0.3 mL of cell-culture derived FAV-HN inactivated vaccine containing 1 × 106 TCID50 virus. Chicken in group B was immunized with same amount of embryo-adapted inactivated vaccine in the same way. Chicken in group C and D were injected with sterile PBS and treated as unvaccinated controls. The SPF chickens were monitored for clinical signs and serum samples were collected from wing veins at 0, 7, 14 and 21 days after immunization.
The FAdV-specific antibodies in serum samples were determined by agar gel diffusion precipitation (AGP) test as described previously [5, 9] and commercially available enzyme-linked immunosorbent assay (ELISA) kit (Biochek, Netherlands) according to manufacturer’s instruction. This ELISA kit has been implemented to detect antibodies against adenovirus group I regardless of serotypes. The sera showing S/P ratios ≥0.5 were considered positive according to manufacturer’s instruction.
Virus challenge study
To evaluate the protective efficacy of two inactivated FAdV-4 vaccines,chicken in groups A, B and C were intramuscularly challenged with virulent FAV-HN virus (107 TCID50/bird). Chickens in group D were not challenged and kept as a sterile control. Clinical signs were observed daily for 2 weeks after challenge. The moribund birds were immediately necropsied to examine gross lesions. Tissue samples of liver were used for histopathology. Two weeks after challenge, all surviving chicken were euthanized and gross lesions were recorded. Protection was assessed by examination of gross lesions on the heart and liver (hydropericardium and hepatitis) and histopathological examination of the liver. All birds experiments were approved by the Institutional Animal Care and Use Committee at National Research Center for Veterinary Medicine and conventional animal welfare regulations and standards were taken into account.
Histopathological studies
Samples of liver from all sacrificed birds were collected and used for histopathological examination. A portion of each tissue sample (0.5–1 cm2) from each tissue sample was fixed in 10% neutral formalin solution for 48 h at room temperature. Tissues were then routinely processed, embedded in paraffin wax by 24 h, and cut into 5 μm sections, following by staining with hematoxylin and eosin. The sections were examined for lesions associated with FAdV infection using light microscopy.
Statistical analysis
One-way ANOVA was performed using Prism 5.0 software package (GraphPad software Inc., San Diego, CA, USA), and a p value of <0.05 was considered statistically significant. Results were expressed as means and standard error of the mean.
Results
Antibody response of vaccinated chicken
Serum antibody against FAdV-4 of the vaccinated birds was measured by AGP test and commercial ELISA. Individual S/P ratio of ELISA for all the sera samples from different groups was calculated and finally the group mean S/P value was estimated.
FAdV-4 antibodies were not detected prior to vaccination and 7 days post-vaccination (dpv) in any of the groups (Table 1). All chicken vaccinated with cell-culture derived FAdV-4 vaccine (group A) showed seroconversion in AGP test and ELISA at 14 dpv. And similar results were obtained at three weeks post vaccination in which 10/10 and 9/10 chicken had seroconversion in AGP and ELISA tests, respectively.
Table 1.
Antibody response in experimental groups measured by agar gel diffusion precipitation (AGP) test and enzyme-linked immunosorbent assay (ELISA)
| Days post vaccination | Cell-culture derived vaccine (A) | Embryo-adapted vaccine (B) | Controls (C and D) | |||
|---|---|---|---|---|---|---|
| AGP testa | ELISAb | AGP testa | ELISAb | AGP testa | ELISAb | |
| 0 | 0/10 | 0/10 | 0/10 | 0/10 | 0/20 | 0/20 |
| 7 | 0/10 | 0/10 | 0/10 | 0/10 | 0/20 | 0/20 |
| 14 | 10/10 | 10/10 | 4/10 | 3/10 | 0/20 | 0/20 |
| 21 | 10/10 | 9/10 | 8/10 | 6/10 | 0/20 | 0/20 |
aNumber of positive serum samples in AGP test/number of tested serum samples
bNumber of positive serum samples in ELISA/number of tested serum samples
Chicken immunized with embryo-adapted inactivated FAdV-4 vaccine (group B) developed lower serum positive rates when compared with the chicken in group A at the corresponding time points. At 14 dpv, 4/10 and 3/10 chickens showed seroconversion in AGP and ELISA tests, respectively and at 21 dpv, 8/10 and 6/10 chicken showed seroconversion in AGP and ELISA tests, respectively (Table 1). No antibodies were detected in unvaccinated chicken (group C and D) (Table 1).
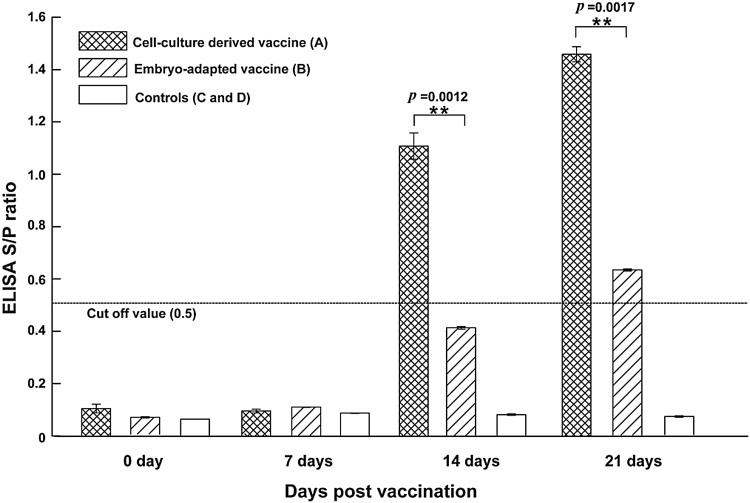
Cell-culture derived FAdV-4 vaccine elicited significantly higher ELISA antibody titers than embryo-adapted vaccine at 14 dpv and 21 dpv (Fig. 1). The S/P ratio of unvaccinated chicken in group C and D did not rise significantly and was below the cutoff value throughout the study (Fig. 1).
Fig. 1.
Antibody response of vaccinated chicken measured by commercial FAdV ELISA kit. Results were indicated as the sample to positive (S/P) ratio of the OD values; S/P ratios ≥0.5 were considered positive. Asterisks (*) marks significant differences between groups (p < 0.05, n = 10). The error bars indicate standard deviation
Challenge protection of vaccinated chickens
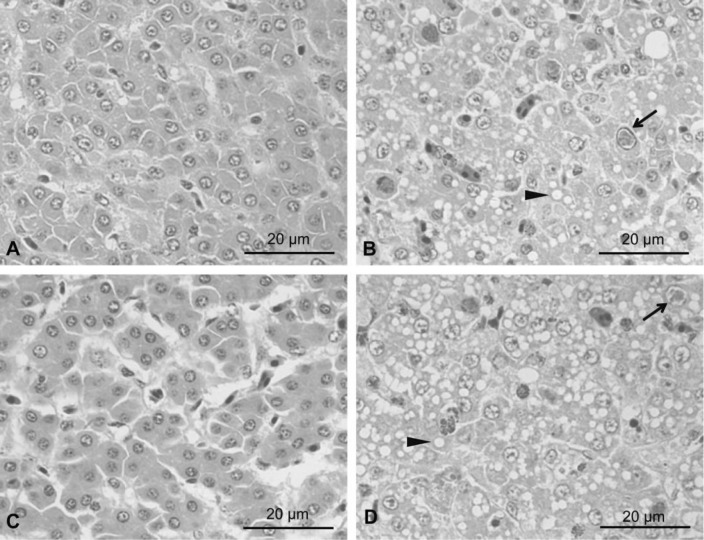
To evaluate protective efficacy of above two vaccines, the vaccinated chicken (groups A and B) and challenge control chicken (group C) were challenged with a virulent FAdV-4 strain (FAV-HN) on 21 dpv. Completely protection without any gross (Fig. 2c) and histopathological lesions (Fig. 3c) was observed in chicken immunized with cell-culture derived FAdV-4 inactivated vaccine (group A; Table 2). By contrast, mild hydropericardium was observed in one out of ten birds in embryo-adapted FAdV-4 inactivated vaccine (group B) after viral challenge (Fig. 2d). Inclusion body hepatitis was observed with intranuclear inclusions and hepatocyte steatosis in liver cells (Fig. 3d). However, there was no death of chicken in both the vaccinated groups.
Fig. 2.
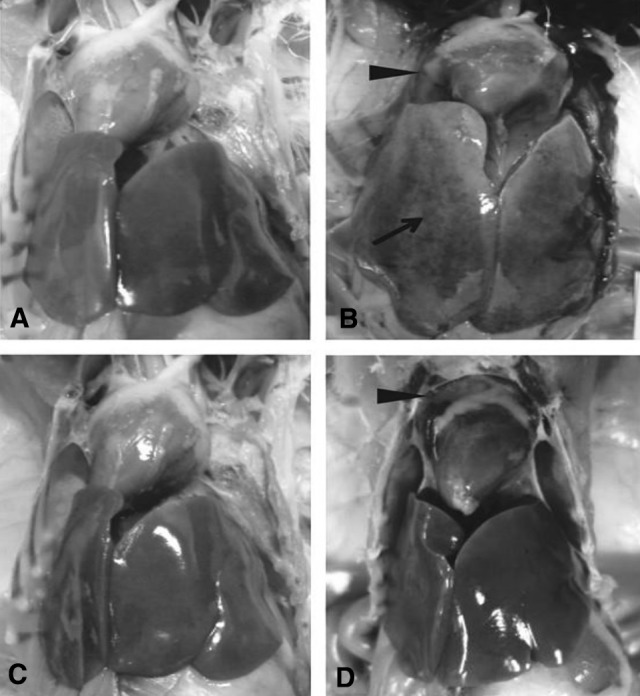
Gross lesions in liver and heart from chicken challenged with FAV-HN strain. The solid arrow indicates hepatitis. The solid triangles indicate hydropericardium. a Liver and heart tissues of unchallenged control chicken. b Liver and heart tissues from the challenged control chicken showing intranuclear inclusions. c Liver and heart tissues of challenged chicken immunized with cell-culture derived inactivated vaccine. d Liver and heart tissues of challenged chicken immunized with embryo-adapted inactivated vaccine
Fig. 3.
Histological changes in liver of chicken challenged with FAV-HN strain. The solid arrows indicate viral inclusion bodies in liver cells. The solid triangles indicate hepatocyte steatosis. a Liver sections of unchallenged control chicken. b Liver sections of challenged control chicken. c Liver sections of challenged chicken immunized with cell-culture derived inactivated vaccine. d Liver sections of challenged chicken immunized with embryo-adapted inactivated vaccine
Table 2.
Protective efficacy of cell-culture derived and embryo-adapted vaccines against FAV-HN challenge
| Group | Mortalitya | Gross lesion | Histological examination | |
|---|---|---|---|---|
| Hydropericardiumb | Hepatitisc | Inclusion bodyd | ||
| Cell-culture derived vaccine (A) | 0/10 | 0/10 | 0/10 | 0/10 |
| Embryo-adapted vaccine (B) | 0/10 | 1/10 | 1/10 | 1/10 |
| Challenge control (C) | 9/10 | 10/10 | 9/10 | 10/10 |
| Sterile control (D) | 0/10 | 0/10 | 0/10 | 0/10 |
aNumber of dead chickens/number of chickens in each group
bNumber of chickens shown hydropericardium/number of necropsied chickens
cNumber of chickens shown enlarged, pale friable liver with hemorrhagic patches/number of necropsied chickens
dNumber of chickens shown intranuclear inclusion body/number of chicks examined
The challenged control chicken in group C showed high mortality (9/10), gross and histopathological lesions (10/10) after challenge (Table 2). All chicken in this group had swollen and yellowish colored liver with necrotic foci, and flabby heart with a severe hydropericardium (Fig. 2b). Liver sections showed intranuclear inclusions and hepatocyte steatosis in liver cells (Fig. 3b). In the sterile control chicken (group D), no gross lesions and histological changes were observed (Figs. 2a, 3a).
Discussion
In recent years, there has been an increase in clinical cases of IBH and HHS all over the world, resulting in considerable economic losses in many countries where the disease has been recorded [6, 10, 13, 14, 21]. The virulent FAdV-4 was reported to be the causative agent for IBH and HHS [7, 11, 18]. Severe FAdV cases with IBH and HHS which occurred typically in 3 to 5-week-old broiler flocks and 10 to 20-week-old layer flocks have been reported in China since 2013 causing huge economic loss in poultry industry [31]. To date, there is no available vaccine to control the FAdV-4 infection in China.
In 2014, a cell-culture derived FAdV-4 inactivated vaccine was reported to provide broad cross-protection against various serotypes of fowl adenovirus [12]. Therefore, the traditional inactivated vaccine based on local strains may work a good choice to control the disease.
In 2013, to make an autologous FAdV vaccine, we isolated twelve virus strains from liver samples of chicken showing IBH and HHS from six provinces (Henan, Shandong, Anhui, Liaoning, Jilin and Hubei) (data not shown). Eleven out of the twelve strains were identified as serotype 4 FAdV by gene sequencing, while only one isolate was identified as serotype 2 FAdV. This indicated that FAdV-4 was the predominant circulating serotype in chicken flocks in China. The above finding was also consistent with previous studies [30]. Among these isolates, FAdV-4 HN strain (FAV-HN) was used to prepare the inactivated vaccines and the challenge virus for this study. FAdV-HN strain proliferated well in LMH cells with high virus titers (>108 TCID50/mL).
Compared to the embryo-adapted inactivated FAdV-4 vaccine, cell-culture derived vaccine elicited earlier and higher antibody responses compared to the embryo-adapted vaccine (Table 1; Fig. 1) which indicated that cell-culture derived vaccine may be a better choice to control this disease. After viral challenge, the histological results further confirmed this conclusion since no histological lesions were observed in any of the chicken in group A but one chicken in group B. After virus challenge, chicken vaccinated with cell-culture derived FAdV-4 vaccine showed no morbidity, mortality or histopathological lesions throughout the experiment (Table 2).
Besides the above immunological and histological advantages of cell-culture derived FAdV4 vaccine, there are also some other well-known advantages of cell-culture derived vaccines over embryo-adapted vaccines. The efficiency of embryo-based production system is limited due to its reliance on a continuous supply of embryonated eggs and production is hard to scale up within a short time to meet changes in demand [22]. Also, propagation of virus on chicken embryo is labor-intensive and time-consuming which is not suitable for the practical production. Cell-based production technology has emerged to overcome the limitations of the egg-based production system. This production system allows manufacturers to respond to pandemic threats faster and more flexible, and supply higher quantities of the vaccines with minimal differences among product batches in a shorter amount of time [8]. And cell-based production system does not introduce new or greater adventitious agents, compared with egg-based vaccine production. Therefore, cell-culture derived FAdV4 vaccine has more practical advantages over the embryo-adapted vaccine.
ELISA is a high sensitive serological method for the detection of serum antibody against FAdV compared to AGP test [29]. However, AGP results showed more positive serea in our study (Table 1). This result could be explained by the antigen used in the commercial ELISA kit which might have different sensitivity to various serotypes of FAdVs. In challenge study, the chicken developing measurable AGP antibody were all completely protected from the adverse effects of the virulent FAV-HN. Two birds in group B immunized with embryo-adapted commercial inactivated FAdV-4 vaccine did not develop a measurable AGP antibody prior to challenge, but one bird was completely protected from the adverse effects of the virulent FAV-HN (Tables 1, 2). A similar phenomenon has been described in previous study [24], in which some birds vaccinated with a live FAdV-4 vaccine are fully protected against a severe challenge even in the absence of measurable neutralizing FAdV-4 antibody. Therefore, besides humoral immune response, cellular immunity could play a very important role in protection to virus infection [24].
In summary, we developed both embryo-adapted and cell-culture derived FAdV-4 inactivated vaccines and compared the antibody responses and protection to a homologous virus challenge on SPF chicken. Our results indicated cell-culture derived vaccine provides better protection than embryo-adapted vaccines based on results of gross pathology and histological examination to control the FAdV infection.
Acknowledgement
This work was supported by Grant from Luoyang Heluo Talent Plan (Dr. Kegong Tian).
Footnotes
Dongying Du, Pantao Zhang, and Xiangdong Li have contributed equally to this study.
Contributor Information
Wujie Liu, Phone: + (86) 10-59198895, Email: jike008@126.com.
Kegong Tian, Phone: + (86) 10-59198895, Email: tiankg@263.net.
References
- 1.Anjum AD, Sabri MA, Iqbal Z. Hydropericarditis syndrome in broiler chickens in Pakistan. Vet Rec. 1989;124(10):247–248. doi: 10.1136/vr.124.10.247. [DOI] [PubMed] [Google Scholar]
- 2.Asthana M, Chandra R, Kumar R. Hydropericardium syndrome: current state and future developments. Adv Virol. 2013;158(5):921–931. doi: 10.1007/s00705-012-1570-x. [DOI] [PubMed] [Google Scholar]
- 3.Benkö MHB, Both GW, Russell WC, Adair BM, Ádám É, et al. Adenoviridae. In: Fauquet CMMM, Maniloff J, Desselberger U, Ball LA, et al., editors. Virus taxonomy, VIIIth report of the international committee on taxonomy of viruses. London: Academic Press; 2005. pp. 213–228. [Google Scholar]
- 4.Chen CW, Lee YP, Wang YF, Yu CK. Formaldehyde-inactivated human enterovirus 71 vaccine is compatible for co-immunization with a commercial pentavalent vaccine. Vaccine. 2011;29(15):2772–2776. doi: 10.1016/j.vaccine.2011.01.094. [DOI] [PubMed] [Google Scholar]
- 5.Cowen BS. A trivalent antigen for the detection of type I avian adenovirus precipitin. Avian Dis. 1987;31(2):351–354. doi: 10.2307/1590884. [DOI] [PubMed] [Google Scholar]
- 6.Dahiya S, Srivastava RN, Hess M, Gulati BR. Fowl adenovirus serotype 4 associated with outbreaks of infectious hydropericardium in Haryana, India. Avian Dis. 2002;46(1):230–3. doi:10.1637/0005-2086(2002)046[0230:FASAWO]2.0.CO;2. [DOI] [PubMed]
- 7.Domanska-Blicharz K, Tomczyk G, Smietanka K, Kozaczynski W, Minta Z. Molecular characterization of fowl adenoviruses isolated from chickens with gizzard erosions. Poult Sci. 2011;90(5):983–989. doi: 10.3382/ps.2010-01214. [DOI] [PubMed] [Google Scholar]
- 8.Dormitzer PR, Tsai TF, Del Giudice G. New technologies for influenza vaccines. Hum Vaccin Immunother. 2012;8(1):45–58. doi: 10.4161/hv.8.1.18859. [DOI] [PubMed] [Google Scholar]
- 9.Dupuy HJ, Blouse LE, Marraro RV. Evaluation of agar-gel double diffusion for the diagnosis of adenovirus infection. Appl Microbiol. 1973;25(6):1013–1014. doi: 10.1128/am.25.6.1013-1014.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomis S, Goodhope AR, Ojkic AD, Willson P. Inclusion body hepatitis as a primary disease in broilers in Saskatchewan, Canada. Avian Dis. 2006;50(4):550–555. doi: 10.1637/7577-040106R.1. [DOI] [PubMed] [Google Scholar]
- 11.Kim JN, Byun SH, Kim MJ, Kim J, Sung HW, Mo IP. Outbreaks of hydropericardium syndrome and molecular characterization of Korean fowl adenoviral isolates. Avian Dis. 2008;52(3):526–530. doi: 10.1637/8178-112207-Case. [DOI] [PubMed] [Google Scholar]
- 12.Kim MS, Lim TH, Lee DH, Youn HN, Yuk SS, Kim BY, et al. An inactivated oil-emulsion fowl Adenovirus serotype 4 vaccine provides broad cross-protection against various serotypes of fowl Adenovirus. Vaccine. 2014;32(28):3564–3568. doi: 10.1016/j.vaccine.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Wang J, Qiu L, Han Z, Liu S. Fowl adenovirus species C serotype 4 is attributed to the emergence of hepatitis–hydropericardium syndrome in chickens in China. Infect Genet Evol. 2016;2016(45):230–241. doi: 10.1016/j.meegid.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Lim TH, Lee HJ, Lee DH, Lee YN, Park JK, Youn HN, et al. Identification and virulence characterization of fowl adenoviruses in Korea. Avian Dis. 2011;55(4):554–560. doi: 10.1637/9730-032011-Reg.1. [DOI] [PubMed] [Google Scholar]
- 15.Lim TH, Kim BY, Kim MS, Jang JH, Lee DH, Kwon YK, et al. Outbreak of gizzard erosion associated with fowl adenovirus infection in Korea. Poult Sci. 2012;91(5):1113–1117. doi: 10.3382/ps.2011-02050. [DOI] [PubMed] [Google Scholar]
- 16.Mansoor MK, Hussain I, Arshad M, Muhammad G. Preparation and evaluation of chicken embryo-adapted fowl adenovirus serotype 4 vaccine in broiler chickens. Trop Anim Health Prod. 2011;43(2):331–338. doi: 10.1007/s11250-010-9694-z. [DOI] [PubMed] [Google Scholar]
- 17.Mittal D, Jindal N, Tiwari AK, Khokhar RS. Characterization of fowl adenoviruses associated with hydropericardium syndrome and inclusion body hepatitis in broiler chickens. Virusdisease. 2014;25(1):114–119. doi: 10.1007/s13337-013-0183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura K, Mase M, Yamaguchi S, Shibahara T, Yuasa N. Pathologic study of specific-pathogen-free chicks and hens inoculated with adenovirus isolated from hydropericardium syndrome. Avian Dis. 1999;43(3):414–423. doi: 10.2307/1592638. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura K, Mase M, Yamaguchi S, Yuasa N. Induction of hydropericardium in one-day-old specific-pathogen-free chicks by adenoviruses from inclusion body hepatitis. Avian Dis. 2000;44(1):192–196. doi: 10.2307/1592524. [DOI] [PubMed] [Google Scholar]
- 20.Niu YJ, Sun W, Zhang GH, Qu YJ, Wang PF, Sun HL, et al. Hydropericardium syndrome outbreak caused by fowl adenovirus serotype 4 in China in 2015. J Gen Virol. 2016 doi: 10.1099/jgv.0.000567. [DOI] [PubMed] [Google Scholar]
- 21.Ono M, Okuda Y, Shibata I, Sato S, Okada K. Reproduction of adenoviral gizzard erosion by the horizontal transmission of fowl adenovirus serotype 1. J Vet Med Sci. 2007;69(10):1005–1008. doi: 10.1292/jvms.69.1005. [DOI] [PubMed] [Google Scholar]
- 22.Partridge J, Kieny MP. Global production capacity of seasonal influenza vaccine in 2011. Vaccine. 2013;31(5):728–731. doi: 10.1016/j.vaccine.2012.10.111. [DOI] [PubMed] [Google Scholar]
- 23.Schachner A, Marek A, Jaskulska B, Bilic I, Hess M. Recombinant FAdV-4 fiber-2 protein protects chickens against hepatitis–hydropericardium syndrome (HHS) Vaccine. 2014;32(9):1086–1092. doi: 10.1016/j.vaccine.2013.12.056. [DOI] [PubMed] [Google Scholar]
- 24.Schonewille E, Jaspers R, Paul G, Hess M. Specific-pathogen-free chickens vaccinated with a live FAdV-4 vaccine are fully protected against a severe challenge even in the absence of neutralizing antibodies. Avian Dis. 2010;54(2):905–910. doi: 10.1637/8999-072309-Reg.1. [DOI] [PubMed] [Google Scholar]
- 25.Shah MS, Ashraf A, Rahman M, Khan MI, Qureshi JA. A subunit vaccine against hydropericardium syndrome using adenovirus penton capsid protein. Vaccine. 2012;30(50):7153–7156. doi: 10.1016/j.vaccine.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Shivachandra SB, Sah RL, Singh SD, Kataria JM, Manimaran K. Immunosuppression in broiler chicks fed aflatoxin and inoculated with fowl adenovirus serotype-4 (FAV-4) associated with hydropericardium syndrome. Vet Res Commun. 2003;27(1):39–51. doi: 10.1023/A:1022058623634. [DOI] [PubMed] [Google Scholar]
- 27.Vera-Hernandez PF, Morales-Garzon A, Cortes-Espinosa DV, Galiote-Flores A, Garcia-Barrera LJ, Rodriguez-Galindo ET, et al. Clinicopathological characterization and genomic sequence differences observed in a highly virulent fowl Aviadenovirus serotype 4. Avian Pathol. 2016;45(1):73–81. doi: 10.1080/03079457.2015.1125443. [DOI] [PubMed] [Google Scholar]
- 28.Winterfield RW, Fadly AM, Gallina AM. Adenovirus infection and disease. I. Some characteristics of an isolate from chickens in Indiana. Avian Dis. 1973;17(2):334–342. doi: 10.2307/1589217. [DOI] [PubMed] [Google Scholar]
- 29.Xie Z, Luo S, Fan Q, Xie L, Liu J, Xie Z, et al. Detection of antibodies specific to the non-structural proteins of fowl adenoviruses in infected chickens but not in vaccinated chickens. Avian Pathol. 2013;42(5):491–496. doi: 10.1080/03079457.2013.829553. [DOI] [PubMed] [Google Scholar]
- 30.Ye J, Liang G, Zhang J, Wang W, Song N, Wang P, et al. Outbreaks of serotype 4 fowl adenovirus with novel genotype, China. Emerg Microbes Infect. 2016;5:e50. doi: 10.1038/emi.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao J, Zhong Q, Zhao Y, Hu YX, Zhang GZ. Pathogenicity and complete genome characterization of fowl adenoviruses isolated from chickens associated with inclusion body hepatitis and hydropericardium syndrome in China. PLoS ONE. 2015;10(7):e0133073. doi: 10.1371/journal.pone.0133073. [DOI] [PMC free article] [PubMed] [Google Scholar]