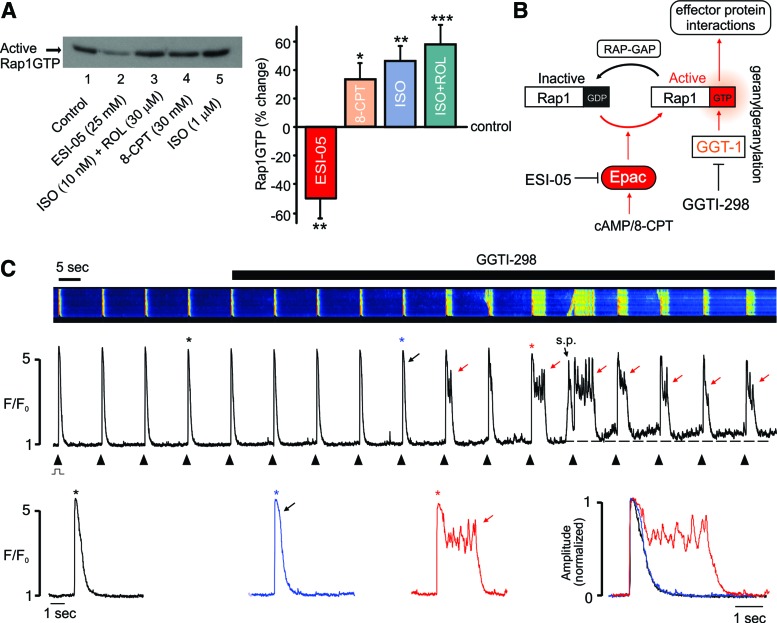
FIG. 2.
Epac2 inhibition decreases basal Rap1GTP and the effects of ESI-05 are mimicked by Rap1GTP inhibition. (A) Original (left) and cumulative data (right) showing that Rap1GTP was detectable under basal conditions (see the Materials and Methods section) in ARVMS and could be reduced by Epac2 inhibition with ESI-05 or increased by the Epac activator 8-CPT (25 μM), ISO (1 μM), or a lower level of ISO (10 nM) in combination with the phosphodiesterase inhibitor, rolipram (20 μM). ISO was added together with the β2 antagonist CI 118,551 (1 μM). n = 4–12, *p < 0.05, **p < 0.01, ***p < 0.005. (B) Schematic showing that Epac activation leads to increased levels of active Rap1GTP and that geranylgeranylation of Rap1GTP by GGT-1 facilitates membrane association and interactions with effector proteins. Inhibition of GGT-1 with GGTI-298 blocks the downstream effects of Rap1GTP. (C) Typical confocal line-scan recordings from a field-stimulated ARVM loaded with fluo-4 (upper), the associated line profile (middle), and selected Ca2+ transients presented on an expended timescale, both individually and normalized/superimposed (lower). Inhibition of GGT-1 with GGTI-298 (0.1 μM) mimicked the effects of ESI-05 on [Ca2+]i transients. s.p.: spontaneous SR Ca2+ release; black arrow: early prolongation of descending phase; red arrows: plateau/Ca2+ oscillations. ‘*’ indicates corresponding Ca2+ transient. Similar effects occurred in 8 of 10 cells from three hearts. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars