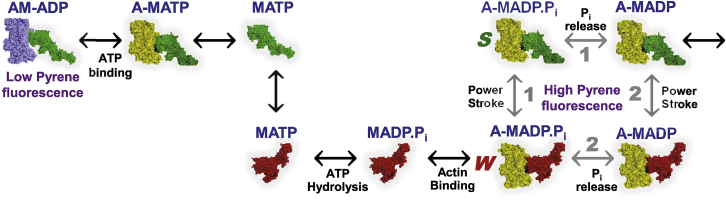
Figure 6.
Shown here is the actin-activated myosin ATPase cycle. In this cycle, changes in the ligand at myosin’s nucleotide site are coupled to changes in actin-binding affinity and conformation between W (lever arm up, red) and S (lever arm down, green). After ATP hydrolysis, release of phosphate (Pi) is associated with the W-to-S transition (power stroke), producing force and movement. Based on kinetics (45) and transient FRET (28) data, the W-S transition for skeletal actomyosin proceeds primarily by pathway 1 (power-stroke before Pi release), whereas β-cardiac actomyosin proceeds by both pathways 1 and 2. The E56G mutation Increases the fraction of the S state, presumably by favoring pathway 1. The color of actin distinguishes those actomyosin states that quench pyrene-actin (blue, leftmost) from those that do not (yellow, all of the rest). To see this figure in color, go online.