Abstract
Mosquito-borne diseases are increasingly being recognized as global threats, with increased air travel accelerating their occurrence in travelers and their spread to new locations. Since the early days of aviation, concern over the possible transportation of infected mosquitoes has led to recommendations to disinsect aircraft. Despite rare reports of mosquitoes, most likely transported on aircraft, infecting people far from endemics areas, it is unclear how important the role of incidentally transported mosquitoes is compared to the role of traveling humans. We used data for Plasmodium falciparum and dengue viruses to estimate the probability of introduction of these pathogens by mosquitoes and by humans via aircraft under ideal conditions. The probability of introduction of either pathogen by mosquitoes is low due to few mosquitoes being found on aircraft, low infection prevalence among mosquitoes, and high mortality. Even without disinsection, introduction via infected human travelers was far more likely than introduction by infected mosquitoes; more than 1000 times more likely for P. falciparum and more than 200 times more likely for dengue viruses. Even in the absence of disinsection and under the most favorable conditions, introduction of mosquito-borne pathogens via air travel is far more likely to occur as a result of an infected human travelling rather than the incidental transportation of infected mosquitoes. Thus, while disinsection may serve a role in preventing the spread of vector species and other invasive insects, it is unlikely to impact the spread of mosquito-borne pathogens.
Author summary
Vector-borne diseases such as malaria, dengue, yellow fever, and Zika are global challenges to public health. International policies, such as the International Health Regulations, call for controlling mosquitoes on aircraft to prevent the introduction of mosquito-borne pathogens and invasive mosquito species. The research presented here used malaria and dengue data to estimate the likelihood of introduction of both pathogens via humans and mosquitoes travelling on airplanes. We found that the probability of introduction of either pathogen by mosquitoes is low due to few mosquitoes being found on aircraft, low infection prevalence among mosquitoes, and a short lifespan. Humans were hundreds of times more likely to spread pathogens via air travel, even in the absence of any mosquito control. Therefore, policies designed to prevent the transportation of infected mosquitoes on airplanes are unlikely to prevent the spread of vector-borne diseases.
Introduction
Mosquito-borne diseases such as malaria [1] and dengue [2] are major causes of morbidity and mortality globally. While these pathogens are endemic only in tropical and subtropical environments, modern air travel has broken down traditional geographic barriers to the extent that infected humans and mosquitoes may quickly travel anywhere in the world [3, 4]. Infected travelers of either variety pose a risk of introducing pathogens to areas where the environment is suitable but the pathogen is not present, leading to local outbreaks. Furthermore, in areas where the pathogen does exist, introductions may result in the replacement of a local strain with a newly introduced one, perhaps with different pathogenicity or drug resistance profiles.
Despite the recognized and realized risk that travelling humans and mosquitoes accidentally transported on aircraft present for the spread of vector-borne diseases [3], there remains a great deal of uncertainty about how best to minimize the spread of pathogens via these two hosts. One option is disinsection, “the procedure whereby health measures are taken to control or kill the insect vectors of human diseases present in baggage, cargo, containers, conveyances, goods and postal parcels.”[5] The broad implementation of such measures has been in place for many decades [6] and is specifically spelled out in the International Health Regulations [5], which state that, “Every conveyance leaving a point of entry situated in an area where vector control is recommended should be disinsected and kept free of vectors.” Effective disinsection could reduce the risk of spread of both specific mosquito species and pathogens [3]. However, specifically for pathogen introduction, it is unclear how important the role of incidentally transported mosquitoes is compared to the role of traveling humans.
Introduction of a pathogen by either a human or a mosquito depends on a sequence of stochastic events. Starting in the source location, there is a probability that a human or mosquito is infected by the pathogen and a probability that a human or mosquito travels to another location. Upon arrival, there is some probability of transmission. For the infected human, this depends on being found by a competent mosquito, being fed upon during the infectious period, having that mosquito first survive the extrinsic incubation period and then transmit the pathogen to another person. For a transported infected mosquito, only the latter part of this sequence is required; the mosquito must survive the extrinsic incubation period and transmit the pathogen to a person before dying.
Here we developed branching process models to compare the risk associated with traveling mosquitoes and traveling humans. Branching process models can be used to explicitly formulate chains of discrete random events such as those described above [7, 8]. In this framework, each stochastic step is first characterized individually and then the sequential series of events is analyzed using well-developed mathematical methods. Here, we are interested in a single outcome, introduced autochthonous transmission, which could result from two alternative pathways, introduction by an infected human or by an infected mosquito.
We focused on two mosquito-borne pathogens of global importance, the malaria parasite P. falciparum and dengue viruses. These pathogens differ in important ways, they are transmitted by different vectors (Anopheles and Aedes mosquitoes, respectively) and have different human infection dynamics (multi-stage, long-term infection or single stage acute infection, respectively). To assess the worst-case scenario for potential introduction, we focused on highly endemic areas where transmission of each pathogen is near optimal and formulated a stochastic description of each step of the composite process using published literature to estimate the key parameters. We then estimated probabilities of introduction via each pathway and assessed the sensitivity of those outcomes to assumptions incorporated into the model.
Materials and methods
We used a branching process framework to model P. falciparum and dengue virus introduction via air travel by either infected mosquitoes or humans. In brief, a branching process is composed of a sequence of stochastic steps, each one taking discrete input from the preceding step and producing discrete stochastic output [7, 8]. The pathogen introduction pathway via mosquitoes included the following steps:
There must be at least one mosquito on the aircraft. We modeled the number of mosquitoes as a Poisson process with mean λM, the average number of mosquitoes found on an aircraft.
There must be at least one infected mosquito. We modeled this step as a Bernoulli process where each mosquito has probability pIM of being infected with the pathogen.
An infected mosquito must transmit the pathogen to at least one human. We modeled this step as a Poisson process, with a mean of R0MH, the average number of humans infected per infected mosquito.
Introduction via humans includes the following steps:
There must be at least one human on the aircraft. We modeled this step as a Poisson process with mean λH, the average number of humans found on an aircraft.
There must be at least one infected human. We modeled this step as a Bernoulli process where each human has a probability pIH of being infected with a pathogen.
An infected human must arrive and transmit the pathogen to at least one mosquito. We modeled this step as a Poisson process, with a mean of R0HM, the average number of mosquitoes infected per infected human.
An infected mosquito must transmit the pathogen to at least one human. We modeled this step as a Poisson process, with a mean of R0MH, the average number of humans infected per infected mosquito. This step is identical to the final step for introduction by a mosquito as the key outcome in both pathways is a human infection.
To capture the full sequence of events in each pathway, we sequentially combined the probability generating functions for each individual step to build a generating function for each complete sequence of events (S1 Text). With these composite functions, we calculated the probability that at least one human transmission event occurred at the destination. This approach enabled the robust consideration of uncertainty and variability in the Poisson and Bernoulli parameters described above; each parameter is considered as a distribution of possible values rather than a fixed value. The key data and assumptions are described below, while further detail is presented in the Supporting Information (S1 Text). Lastly, we assessed the sensitivity of the branching process model results to parameter assumptions for each individual step by estimating the relative change in introduction probabilities per unit relative change in each parameter (S1 Text).
Travel
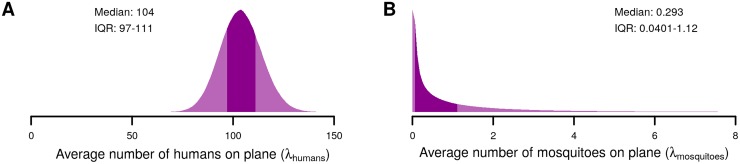
The average number of human passengers on flights globally was 104 in 2015 according to the International Civil Aviation Organization, which we assumed to be Poisson distributed (Fig 1A) (http://www.icao.int/annual-report-2015/Documents/Appendix_1_en.pdf). Approximately 0.91 mosquitoes (95%CI: 0.00009–5.3) were found per aircraft on average across 17 studies of 559,579 aircraft from 1931 to 1999 [9–25] (Fig 1B). Because of the variety of aircraft, time periods, locations, objectives, methods, and data reported in these studies, we made the conservative assumption that all mosquitoes were competent vectors, female, and alive, and therefore capable of being infected and transmitting each pathogen. This scenario should therefore overestimate vector mosquito transportation by aircraft.
Fig 1. Distributions of the parameters for the average number of humans (A) and mosquitoes (B) on aircraft.
The dark shaded areas in each distribution indicate the interquartile range. The median and interquartile range (IQR) of each distribution is shown in the corresponding panel.
Plasmodium falciparum
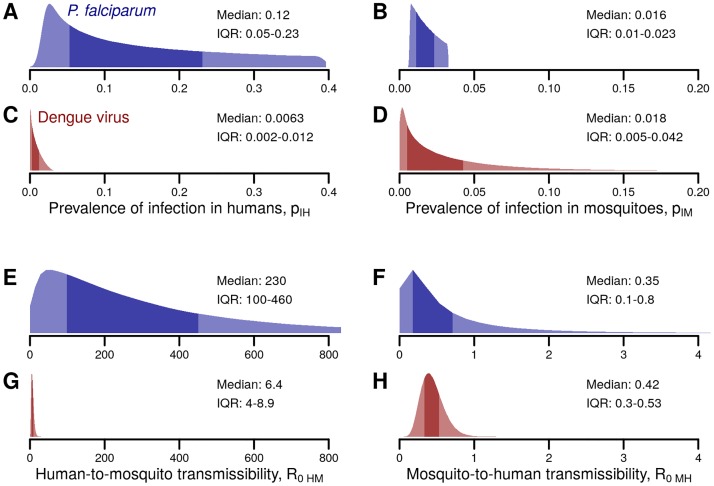
Estimates of the prevalence in humans were based on over 3,000 surveys of 2–10 year olds [26]. The prevalence of P. falciparum reported in these surveys ranged from 0% to almost 100%, with a mean of 23% (Fig 2A). Fewer data were available for mosquito populations, but the estimated prevalence of sporozoites in mosquitoes across 41 studies ranged from 0.01% to 7.6% with a mean of 2.3% [27] (Fig 2B). Because our focus was the relative probability of introduction by each pathway, we reduced extreme variability by sampling only from the interquartile range of the distributions of pIH and pIM. Finally, to account for correlation between human and mosquito prevalence when comparing the two pathways, the samples were split into deciles and randomly paired within each decile, such that samples with relatively high human infection prevalence also had relatively high vector infection prevalence.
Fig 2. Distributions of parameters in the branching process for P. falciparum and dengue virus introduction.
The prevalence of infection in humans and mosquitoes is characterized by Bernoulli distributions with parameters pIH (A and C) and pIM (B and D), respectively. Transmissibility for humans to mosquitoes and mosquitoes to humans are characterized by Poisson distributions with parameters R0HM (E and G) and R0MH (F and H), respectively. The dark shaded areas in each distribution indicate the interquartile range. Each panel provides the median and the interquartile range (IQR) of the corresponding distribution.
R0HM was calculated as the product of the mosquito density r (mean: 31 mosquitoes per person [27]), mosquito biting rate b (above), the human-to-mosquito transmissibility pHM (mean: 0.16 [1]), and the duration of infectiousness D (mean: 205 days [28]) (Fig 2E):
R0MH was calculated using the classic formulation [29]:
accounting for the mosquito biting rate b (mean: 0.4 bites per day [27]), the mosquito-to-human transmissibility pMH (mean: 0.55 [1], the mosquito mortality rate μ (mean: 0.13 per day [27]), and the length of the extrinsic incubation period, EIP (mean: 10.9 days [27]) (Fig 2F).
Dengue virus
Estimates of the prevalence of DENV infection in humans were based on twenty seroprevalence studies (primarily in children) in hyperendemic locations across the globe [30–49]. The mean yearly infection rate ranged from 2% to 90%, averaging approximately 23%. To estimate the average daily prevalence of incubating and infectious humans, we sampled the duration of infection as the sum of the intrinsic incubation period (IIP, mean: 5.9 days [50]) and the adjusted infectious period (D, mean: 5.0 days, see below) discounted by the overlap (O, approximately 1 day [51]), and multiplied yearly incidence by (IIP + D–O)/365. The mean prevalence of DENV infection in humans was approximately 0.08% (Fig 2C). Mosquito infection rates were estimated from 13 studies in areas with ongoing dengue outbreaks that provided either direct measurements of infection rates, minimum infection rates through pooled samples or indirect measurements of infection rates via maximum likelihood estimates of pooled samples [52–64]. The mean prevalence of DENV infection in mosquitoes was 3% (Fig 2D). As for malaria, we sampled from the interquartile ranges of each distribution and stratified sampled human and vector prevalence by deciles.
R0MH and R0HM for DENV (Fig 2G and 2H) were estimated as for malaria (above) under the assumption that temperature was approximately 30°C, i.e. conducive to efficient dengue transmission [65]. The mean mosquito biting rate b was 0.7 bites per day [66], the mean mosquito-to-human transmissibility pMH was 0.5, the mean mosquito mortality rate μ was 0.21 per day [67], and the mean length of the extrinsic incubation period, EIP was 6.5 days [50], and the mean mosquito density r was 2 mosquitoes per person [68]. To obtain the dengue parameter R0HM, we do not estimate the parameters pHM and D independently, as was done for malaria. Rather, we obtain them in aggregate as the ‘Human Total Infectiousness’ (HTI), by integrating a logistic function of the human-to-mosquito transmissibility over the course of infection [69].
Results
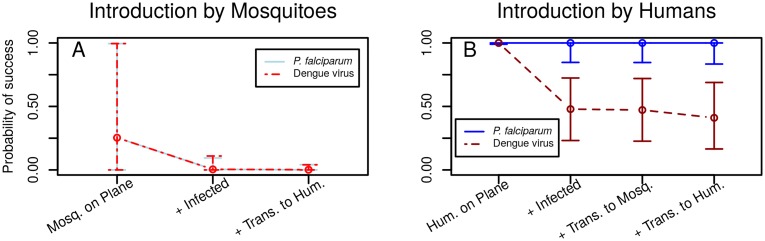
The stepwise probabilities of P. falciparum or dengue virus introduction were quantitatively nearly identical for the mosquito pathway (Fig 3A). The probability of a mosquito being on an aircraft was generally low but variable (median: 0.25, 95% Credible Interval (CI): 9×10−5–0.995). The median probability of at least one mosquito on board being infected was much lower, less than 0.005 for both pathogens and the median probability for an infected mosquito traveling and subsequently transmitting to a human was less than 0.002. Humans, in contrast, were always present on aircraft and had high probabilities of completing each subsequent step of the introduction pathway: at least one traveler being infected, at least one instance of transmission to a mosquito, and at least one instance of transmission to a human (Fig 3B). For traveling humans, the median probability of a local transmission event was approximately 1.0 and 0.4 for P. falciparum and dengue viruses, respectively.
Fig 3. Step by step probabilities of success for each introduction pathway.
For each sequential step in the mosquito (A) and human (B) introduction processes, we calculated the median (points) and 95% Credible Interval (vertical lines) for the probability of at least one event occurring. For example, the median probability that at least one human is on an aircraft is approximately 1.0 while the median probability that there is at least one human on an aircraft AND who is infected with a dengue virus is approximately 0.5. Note that for the introduction pathway via infected mosquitoes, the probabilities of success at each step (as well as the 95% CI) are nearly identical for both malaria and dengue.
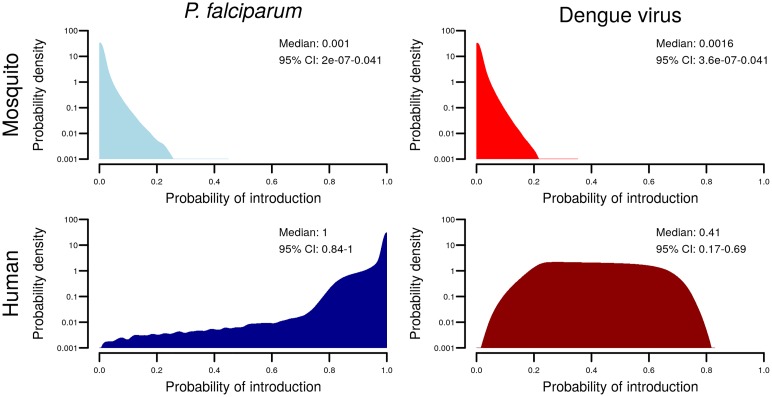
Overall, the median probability that a single aircraft traveling from an endemic area to another highly suitable area would lead to autochthonous human transmission of P. falciparum due to incidental transportation of mosquitoes was 0.001 (95% CI: 0.000–0.041). In contrast, the median probability of introduction by infected human travelers was 1.00 (95% CI: 0.84–1.00). For dengue viruses, the probabilities were 0.002 (95% CI: 0.00–0.04) for mosquitoes and 0.41 (95% CI: 0.17–0.69) for humans (Fig 4). The average odds of introduction by humans versus mosquitoes was 1,000:1 (95% CI: 500:1–1,800:1) for malaria and 240:1 (95% CI: 150:1–370:1) for dengue. For details on how these were obtained, see S1 Text.
Fig 4. Distributions for the probability of introduction by each pathway.
The density (log scale) for the probability of introduction via each pathway across 1 million simulations for P. falciparum (left column) and dengue virus (right column) and for the two pathways of introduction: infected mosquitoes (top row) and infected humans (bottom row). Each panel provides the median and 95% credible interval.
The difference in introduction probabilities between the mosquito and human pathways starts with different probabilities of travel; multiple humans are found on most aircraft, while mosquitoes are relatively rare. For malaria, infection was more prevalent in humans (mean: 0.15) than in mosquitoes (mean: 0.02), so that the median estimated numbers of malaria infected humans and mosquitoes on an aircraft were 12 (95% CI: 2–40) and 0.005 (95% CI: ~1.4×10−6–0.1), respectively. The estimated probability of having an infected human onboard was approximately 200 (95% CI: 10–1×106) times higher than the probability of an infected mosquito being onboard. For dengue, the probability of being infected was higher for mosquitoes in the midst of an outbreak (mean: 0.03) compared to average human prevalence over time periods encompassing outbreaks (mean: 0.007). Nevertheless, the difference in frequency of travel still translates to the probability of infected human travelers being approximately 93 (95% CI: 5–4.0×105) times higher than the probability of infected mosquitoes being on an aircraft.
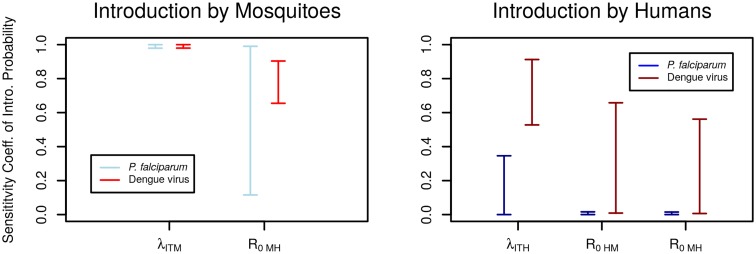
We assessed the importance of each pathway-specific sub-component by estimating sensitivity coefficients (Fig 5). Sensitivity related to the prevalence of infection is intrinsically linked to the number of mosquitoes or humans on an aircraft because both contribute to the mean number of infected individuals onboard, λITM and λITH, respectively. The mosquito pathway was highly sensitive to λITM for both pathogens, a 1% change in either the number of mosquitoes or the prevalence of infection in mosquitoes resulted in a change of approximately 1% in the probability of introduction. The importance of R0MH was lower, with a mean change of approximately 0.8% in introduction probability per 1% change in R0MH. Sensitivity for the human introduction pathway varied between malaria and dengue: P. falciparum introduction was mostly insensitive to parameter changes as introduction was highly probable across the parameter space while dengue showed some sensitivity, especially to the number of humans on an aircraft and the prevalence of infection (combined in λITH).
Fig 5. Sensitivity of introduction pathways to each individual step.
Sensitivity coefficients indicate the percent change in the probability of introduction given a 1% change in each individual parameter (S1 Text). The probability of travel and the prevalence of infection are combined in the parameters λITM and λITH.
Discussion
Mosquito-borne diseases such as malaria, dengue, yellow fever, chikungunya, and Zika are all endemic in tropical or subtropical areas. These diseases have also been documented among travelers and pose a transmission risk in areas where the pathogen is absent but the relevant mosquito vector is present, as has seen with chikungunya and Zika viruses in recent years. This risk is also a serious concern for areas where interventions are being targeted to prevent the invasion of drug resistant pathogens or to eliminate a pathogen altogether, such as current efforts to curb malaria [70, 71]. We assessed the relative risk of vector-borne pathogen introduction by infected humans and infected mosquitoes aboard aircraft, specifically estimating the probability of introduction of P. falciparum and dengue viruses. To clarify the relative risks of the two alternative pathways we focused on a situation that would favor introduction, in which the origin of air travel was assumed to be a highly endemic location and the destination was assumed to be equally suitable for transmission but nevertheless free of the pathogen. Introduction via infected human travelers was far more likely than introduction via infected mosquitoes; more than 1000 times more likely for P. falciparum and more than 200 times more likely for dengue viruses.
The low probability of introduction by mosquitoes stems from three key components. First, mosquitoes are rarely found on aircraft; the majority of aircraft from the 17 surveys had no mosquitoes on them and the highest number of vector species reported on a single aircraft was 17 Anopheles gambiae mosquitoes (possibly including both males and females) [13]. Second, if mosquitoes do make it onto aircraft, they are unlikely to be infected; the estimated infection prevalence of infection in mosquitoes was generally well below 5% even under the optimal conditions assessed here. The importation of P. falciparum or dengue virus via mosquitoes only becomes likely in the event that both of these rare conditions occur together. Finally, for a human to become infected at the destination, an imported, infected mosquito must survive long enough to complete the incubation period and feed on another human. Even in the highly endemic settings considered here, most infected mosquitoes do not survive long enough to transmit the pathogen.
The introduction probabilities differed between the two pathogens primarily because of the difference in infectious period (approximately 205 days for P. falciparum versus approximately 5 days for dengue viruses), which imparts increased human infection prevalence and increased human to mosquito transmission. Despite this difference and an estimate of higher prevalence in mosquitoes than humans, the relative risk for human introduction was still 200 times higher than for introduction by mosquitoes. This supports the generalizability of the finding that the frequency of travel and the differential transmissibility (human-to-mosquito being higher than mosquito-to-human) drive the difference in introduction risk.
The estimates provided here reflect a worst-case scenario in which prevalence is high in the source location and transmission is highly likely in the destination location. This choice was made to explicitly focus on the relative probability of introduction via human or mosquitoes as opposed to the absolute probability. In more realistic situations, the risk of introduction by either humans or mosquitoes is likely to be much smaller due to a number of factors influencing the association between infection risk and travel likelihood. For example, infection risk may be lower among traveling humans compared to the general population because they may have different exposure risk (e.g. if staying in an air-conditioned hotel) or different infection risk (e.g. adults are more likely to be immune to DENV infection). To assess how this might change the relative probability of introduction, we simulated a 90% reduction in human infection prevalence. Even under this condition, human travelers were more likely to introduce both pathogens than accidentally transported mosquitoes (S1 Text). Moreover, airports are often far from rural areas with high P. falciparum prevalence, so the prevalence of infection in both humans and mosquitoes on aircraft is likely much lower than estimated here. And efforts to limit transmission further reduce this risk [72]. The frequency of vector mosquitoes being accidentally transported on aircraft was also likely overestimated as we used average numbers of all mosquitoes regardless of species, sex, and viability (many were reported dead in the studies). These factors all suggest that we have overestimated the risk of introduction, especially by mosquitoes. However, the differences in transmissibility and human travel likelihood would change little, so the risk posed by travelling humans remains substantially higher than the risk posed by the accidental transportation of mosquitoes.
Recommendations for disinsection targeted at mosquitoes of public health importance focus on safety, effectiveness of the disinsection process, and the prevention of the introduction of invasive mosquito species and invasive pathogens via infected mosquitoes [3, 73]. The safety of passengers, crew, and the physical aircraft are key challenges not addressed here [73]. Effectiveness of the disinsection process itself is an additional concern; methods and implementation of disinsection vary, few insecticides are approved for use on aircraft, and many mosquitoes are resistant to some or all of those insecticides [73]. Safe and effective disinsection may help reduce the threat of vector mosquito invasion via aircraft to areas where those mosquitoes do not already exist [73], though some key vector species (e.g. Ae. aegypti) are already widely distributed and introduction has most often been attributed to shipping [74–76]. The importation of vector mosquito species was not addressed further here as this work was limited to the risk of mosquito-borne pathogen introduction. This introduction can manifest as a single transmission event (e.g. airport malaria or airport dengue) or the more problematic initiation of local transmission. Our results show that even in the absence of disinsection and under the most favorable conditions, the probability of any transmission resulting from the introduction of an infected mosquito by aircraft is very low. Moreover, the risk of introduced transmission via human travelers is 2–3 orders of magnitude higher. Cases of reported airport malaria or dengue number in the single digits per year [3, 77, 78], supporting the assertion that while infected mosquitoes on aircraft may transmit pathogens, it is extremely rare. Meanwhile hundreds to thousands of infections in human travelers are reported each year [79–84] each of which provides a higher likelihood of initiating transmission where vectors are present.
Concern about the spread of vector-borne pathogens via mosquitoes on aircraft has existed almost as long as aircraft themselves [9]. That concern continues to grow with the drastic increase in air travel and the arbovirus pandemics of recent years. Vector-borne pathogens are named as such for their dependence on insect vectors to complete the transmission cycle. However, on an international scale, clearly vector-borne diseases spread via humans travelling on aircraft, rather than insects. Even with perfect disinsection, which is far from guaranteed, the likely impact of disinsection on the spread of vector borne disease is negligible as that spread is many times more likely to occur due to human travel.
Supporting information
(PDF)
Data Availability
The manuscript relies entirely on data from previously published studies cited in the manuscript. Key estimates derived from the original data are reported in the main text and supporting information.
Funding Statement
AJT was supported by funding from NIH/NIAID (U19AI089674), the Bill & Melinda Gates Foundation (OPP1106427, 1032350, OPP1134076), the Clinton Health Access Initiative, and a Wellcome Trust Sustaining Health Grant (106866/Z/15/Z). MAJ received partial support from the Models of Infectious Disease Agent Study program (Cooperative Agreement 1U54GM088558). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gething P. W. et al. , A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J 10, 378 (2011). doi: 10.1186/1475-2875-10-378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt S. et al. , The global distribution and burden of dengue. Nature 496, 504–507 (2013). doi: 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gratz N. G., Steffen R., Cocksedge W., Why aircraft disinsection? Bull. World Health Organ. 78, 995–1004 (2000). [PMC free article] [PubMed] [Google Scholar]
- 4.Tatem A. J. et al. , Air travel and vector-borne disease movement. Parasitology 139, 1816–1830 (2012). doi: 10.1017/S0031182012000352 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization, "International Health Regulations (2005) Second Edition," (World Health Organization, Geneva, 2008). [Google Scholar]
- 6.Duguet J., Disinsectization of aircraft; study made in connexion with the revision of international conventions. Bull. World Health Organ. 2, 155–191 (1949). [PMC free article] [PubMed] [Google Scholar]
- 7.T. Harris. (Prentice-Hall Inc).
- 8.Athreya K. B., A simple proof of a result of Kesten and Stigum on supercritical multitype Galton-Watson branching process. The Annals of Mathematical Statistics, 195–202 (1970). [Google Scholar]
- 9.Griffitts T., Griffitts J., Mosquitoes Transported by Airplanes: Staining Method Used in Determining Their Importation. Public Health Reports (1896–1970), 2775–2782 (1931). [Google Scholar]
- 10.Sice A., Sautet J., Ethes Y., One of the most significant vectors of malaria in Africa, Anopheles gambiae Giles, 1903. Can it be transported into France on aircraft? Revue Mé decine Hygiene Tropicale 31, 137 (1939). [Google Scholar]
- 11.Welch E., Insects found on aircraft at Miami, Fla., in 1938. Pub Hlth Rep 54, 561–566 (1939). [Google Scholar]
- 12.Pemberton C., Insects carried in transpacific Airplanes. A Review of Quarantine Work prior to December 7, 1941. Hawaiian Planters' Record 48, 183–186. (1944). [Google Scholar]
- 13.CARNEIRO d. M. F., Cerqueira N., Insects and other arthropods captured by the Brazilian sanitary service on landplanes or seaplanes arriving in Brazil between January 1942 and December 1945. Boletín de la Oficina Sanitaria Panamericana. Pan American Sanitary Bureau 26, 22 (1947). [PubMed] [Google Scholar]
- 14.Hughes J. H., Aircraft and Publie Health Service. Foreign Quarantine Entomology. Public Health Rep., (1949). [Google Scholar]
- 15.Laird M., Insects collected from Aircraft arriving in New Zealand from Abroad. Zool. Publ. Victoria Univ. College., (1951). [Google Scholar]
- 16.Laird M., Insects collected from aircraft arriving in New Zealand during 1951. The Journal of aviation medicine 23, 280 (1952). [PubMed] [Google Scholar]
- 17.Hughes J. H., Mosquito interceptions and related problems in aerial traffic arriving in the United States. Mosquito News 21, 93–100 (1961). [Google Scholar]
- 18.EVANS B. R., JOYCE C. R., PORTER J. E., Mosquitoes and other arthropods found in baggage compartments of international aircraft. Mosquito News 23, 9–12 (1963). [Google Scholar]
- 19.Basio R., Prudencio M., Chanco I., Notes on the aerial transportation of mosquitoes and other insects at the Manila International Airport. Philippine Entomologist 1, 407–408 (1970). [Google Scholar]
- 20.Highton R., van Someren E., The transportation of mosquitos between international airports. Bull. World Health Organ. 42, 334 (1970). [PMC free article] [PubMed] [Google Scholar]
- 21.Ogata K., Tanaka I., Ito Y., Morii S., Survey of the medically important insects carried by the int ernational aircrafts to Tokyo International Airport. Jap J Sanit Zool, (1974). [Google Scholar]
- 22.Le Maitre A., Chadee D. D., Arthropods collected from aircraft at Piarco International Airport, Trinidad, West Indies. Mosquito News 43, 21–23 (1983). [Google Scholar]
- 23.R. Russell, N. Rajapaksa, P. Whelan, W. Langsford, Mosquito and other insect introductions to Australia aboard international aircraft, and the monitoring of disinsection procedures. (1984).
- 24.Takahashi S., Laird M., Survey on accidental introductions of insects entering Japan via aircraft Commerce and the spread of pests and disease vectors., 65–79 (1984). [Google Scholar]
- 25.Dobbs T. T., Brodel C. F., Cargo aircraft as a pathway for the entry of nonindigenous pests into south Florida. Florida Entomologist 87, 65–78 (2004). [Google Scholar]
- 26.Guerra C. A. et al. , The limits and intensity of Plasmodium falciparum transmission: implications for malaria control and elimination worldwide. PLoS Med. 5, e38 (2008). doi: 10.1371/journal.pmed.0050038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Killeen G. F., Ross A., Smith T., Infectiousness of malaria-endemic human populations to vectors. Am. J. Trop. Med. Hyg. 75, 38–45 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Sama W., Owusu-Agyei S., Felger I., Dietz K., Smith T., Age and seasonal variation in the transition rates and detectability of Plasmodium falciparum malaria. Parasitology 132, 13–21 (2006). doi: 10.1017/S0031182005008607 [DOI] [PubMed] [Google Scholar]
- 29.Anderson R. M., May R. M., Infectious diseases of humans: dynamics and control Oxford science publications (Oxford University Press, Oxford; New York, 1991), pp. viii, 757 p. [Google Scholar]
- 30.Anderson K. B. et al. , Burden of symptomatic dengue infection in children at primary school in Thailand: a prospective study. The Lancet 369, 1452–1459 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Balmaseda A. et al. , Trends in patterns of dengue transmission over 4 years in a pediatric cohort study in Nicaragua. J. Infect. Dis. 201, 5–14 (2010). doi: 10.1086/648592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Comach G. et al. , Dengue virus infections in a cohort of schoolchildren from Maracay, Venezuela: a 2-year prospective study. Vector-Borne and Zoonotic Diseases 9, 87–92 (2009). doi: 10.1089/vbz.2007.0213 [DOI] [PubMed] [Google Scholar]
- 33.Burke D. S., Nisalak A., Johnson D. E., Scott R., A prospective study of dengue infections in Bangkok. The American journal of tropical medicine and hygiene 38, 172–180 (1988). [DOI] [PubMed] [Google Scholar]
- 34.Endy T. P. et al. , Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am. J. Epidemiol. 156, 40–51 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Graham R. et al. , A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia I. studies in 1995–1996. The American journal of tropical medicine and hygiene 61, 412–419 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Porter K. R. et al. , Epidemiology of dengue and dengue hemorrhagic fever in a cohort of adults living in Bandung, West Java, Indonesia. The American journal of tropical medicine and hygiene 72, 60–66 (2005). [PubMed] [Google Scholar]
- 37.Yoon I.-K. et al. , Underrecognized mildly symptomatic viremic dengue virus infections in rural Thai schools and villages. J. Infect. Dis. 206, 389–398 (2012). doi: 10.1093/infdis/jis357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teixeira M. d. G. et al. , Dynamics of dengue virus circulation: a silent epidemic in a complex urban area. Trop. Med. Int. Health 7, 757–762 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Honorio N. A. et al. , Spatial evaluation and modeling of dengue seroprevalence and vector density in Rio de Janeiro, Brazil. PLoS Neglected Tropical Diseases 3, e545 (2009). doi: 10.1371/journal.pntd.0000545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.SANGKAWIBHA N. et al. , Risk factors in dengue shock syndrome: a prospective epidemiologic study in Rayong, Thailand I. The 1980 outbreak. Am. J. Epidemiol. 120, 653–669 (1984). [DOI] [PubMed] [Google Scholar]
- 41.Libraty D. H. et al. , A prospective nested case-control study of Dengue in infants: rethinking and refining the antibody-dependent enhancement dengue hemorrhagic fever model. PLoS Med. 6, e1000171 (2009). doi: 10.1371/journal.pmed.1000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capeding R. Z. et al. , The incidence, characteristics, and presentation of dengue virus infections during infancy. The American journal of tropical medicine and hygiene 82, 330–336 (2010). doi: 10.4269/ajtmh.2010.09-0542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrison A. C. et al. , Epidemiology of dengue virus in Iquitos, Peru 1999 to 2005: interepidemic and epidemic patterns of transmission. PLoS Negl Trop Dis 4, e670 (2010). doi: 10.1371/journal.pntd.0000670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waterman S. et al. , Dengue transmission in two Puerto Rican communities in 1982. The American journal of tropical medicine and hygiene 34, 625–632 (1985). [DOI] [PubMed] [Google Scholar]
- 45.Tissera H. et al. , Burden of dengue infection and disease in a pediatric cohort in urban Sri Lanka. The American journal of tropical medicine and hygiene 91, 132–137 (2014). doi: 10.4269/ajtmh.13-0540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tien N. T. K. et al. , A prospective cohort study of dengue infection in schoolchildren in Long Xuyen, Viet Nam. Trans. R. Soc. Trop. Med. Hyg. 104, 592–600 (2010). doi: 10.1016/j.trstmh.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 47.Reyes M. et al. , Index cluster study of dengue virus infection in Nicaragua. The American journal of tropical medicine and hygiene 83, 683–689 (2010). doi: 10.4269/ajtmh.2010.10-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beckett C. G. et al. , Early detection of dengue infections using cluster sampling around index cases. The American journal of tropical medicine and hygiene 72, 777–782 (2005). [PubMed] [Google Scholar]
- 49.Sabchareon A. et al. , Dengue infection in children in Ratchaburi, Thailand: a cohort study. I. Epidemiology of symptomatic acute dengue infection in children, 2006–2009. PLoS Negl Trop Dis 6, e1732 (2012). doi: 10.1371/journal.pntd.0001732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan M., Johansson M. A., The incubation periods of dengue viruses. PLoS One 7, e50972 (2012). doi: 10.1371/journal.pone.0050972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishiura H., Halstead S. B., Natural history of dengue virus DENV-1 and DENV-4 infections: Reanalysis of classic studies. J. Infect. Dis. 195, 1007–1013 (2007). doi: 10.1086/511825 [DOI] [PubMed] [Google Scholar]
- 52.Chow V. et al. , Monitoring of dengue viruses in field-caught Aedes aegypti and Aedes albopictus mosquitoes by a type-specific polymerase chain reaction and cycle sequencing. The American journal of tropical medicine and hygiene 58, 578–586 (1998). [DOI] [PubMed] [Google Scholar]
- 53.Chung Y. K., Pang F. Y., Dengue virus infection rate in field populations of female Aedes aegypti and Aedes albopictus in Singapore. Trop. Med. Int. Health 7, 322–330 (2002). [DOI] [PubMed] [Google Scholar]
- 54.Garcia-Rejon J. et al. , Dengue virus—infected Aedes Aegypti in the home environment. The American journal of tropical medicine and hygiene 79, 940–950 (2008). [PubMed] [Google Scholar]
- 55.García-Rejón J. E. et al. , Mosquito infestation and dengue virus infection in Aedes aegypti females in schools in Merida, Mexico. The American journal of tropical medicine and hygiene 84, 489–496 (2011). doi: 10.4269/ajtmh.2011.10-0654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Méndez F. et al. , Human and mosquito infections by dengue viruses during and after epidemics in a dengue—endemic region of Colombia. The American journal of tropical medicine and hygiene 74, 678–683 (2006). [PubMed] [Google Scholar]
- 57.Urdaneta L. et al. , Detection of dengue viruses in field-caught Aedes aegypti (Diptera: Culicidae) in Maracay, Aragua state, Venezuela by type-specific polymerase chain reaction. Infection, Genetics and Evolution 5, 177–184 (2005). doi: 10.1016/j.meegid.2004.09.004 [DOI] [PubMed] [Google Scholar]
- 58.Ritchie S. A., Long S., Smith G., Pyke A., Knox T. B., Entomological investigations in a focus of dengue transmission in Cairns, Queensland, Australia, by using the sticky ovitraps. J. Med. Entomol. 41, 1–4 (2004). [DOI] [PubMed] [Google Scholar]
- 59.Romero-Vivas C., Leake C., Falconar A., Determination of dengue virus serotypes in individual Aedes aegypti mosquitoes in Colombia. Med. Vet. Entomol. 12, 284–288 (1998). [DOI] [PubMed] [Google Scholar]
- 60.Chen C. F. et al. , Screening of dengue virus in field-caught Aedes aegypti and Aedes albopictus (Diptera: Culicidae) by one-step SYBR green-based reverse transcriptase-polymerase chain reaction assay during 2004–2007 in Southern Taiwan. Vector Borne Zoonotic Dis 10, 1017–1025 (2010). doi: 10.1089/vbz.2008.0069 [DOI] [PubMed] [Google Scholar]
- 61.Angel B., Joshi V., Distribution of dengue virus types in Aedes aegypti in dengue endemic districts of Rajasthan, India. (2009). [PubMed] [Google Scholar]
- 62.Kihara Y. et al. , Rapid determination of viral RNA sequences in mosquitoes collected in the field. J. Virol. Methods 146, 372–374 (2007). doi: 10.1016/j.jviromet.2007.07.008 [DOI] [PubMed] [Google Scholar]
- 63.Tuksinvaracharn R. et al. , Prevalence of dengue virus in Aedes mosquitoes during dry season by semi-nested reverse transcriptase-polymerase chain reaction (semi-nested RT-PCR). J. Med. Assoc. Thai. 87, 129–133 (2004). [PubMed] [Google Scholar]
- 64.Ilkal M. A. et al. , Entomological investigations during outbreaks of dengue fever in certain villages in Maharashtra state. Indian J. Med. Res. 93, 174–178 (1991). [PubMed] [Google Scholar]
- 65.Brady O. J. et al. , Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasites & vectors 7, 338 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scott T. W. et al. , Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: blood feeding frequency. J. Med. Entomol. 37, 89–101 (2000). [DOI] [PubMed] [Google Scholar]
- 67.Brady O. J. et al. , Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasites & vectors 6, 351 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Focks D. A., Brenner R. J., Hayes J., Daniels E., Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am. J. Trop. Med. Hyg. 62, 11–18 (2000). [PubMed] [Google Scholar]
- 69.Nguyet M. N. et al. , Host and viral features of human dengue cases shape the population of infected and infectious Aedes aegypti mosquitoes. Proc. Natl. Acad. Sci. U. S. A. 110, 9072–9077 (2013). doi: 10.1073/pnas.1303395110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chiyaka C. et al. , The stability of malaria elimination. Science 339, 909–910 (2013). doi: 10.1126/science.1229509 [DOI] [PubMed] [Google Scholar]
- 71.Smith D. L. et al. , A sticky situation: the unexpected stability of malaria elimination. Phil. Trans. R. Soc. B 368, 20120145 (2013). doi: 10.1098/rstb.2012.0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bhatt S. et al. , The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211 (2015). doi: 10.1038/nature15535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.World Health Organization, "Report of the WHO Ad-hoc Advisory Group on aircraft disinsection for controlling the international spread of vectorborne diseases," (World Health Organization, Geneva, 2016). [Google Scholar]
- 74.Lounibos L. P., Invasions by insect vectors of human disease. Annu. Rev. Entomol. 47, 233–266 (2002). doi: 10.1146/annurev.ento.47.091201.145206 [DOI] [PubMed] [Google Scholar]
- 75.Tatem A. J., Hay S. I., Rogers D. J., Global traffic and disease vector dispersal. Proceedings of the National Academy of Sciences 103, 6242–6247 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Randolph S. E., Rogers D. J., The arrival, establishment and spread of exotic diseases: patterns and predictions. Nature Reviews Microbiology 8, 361–371 (2010). doi: 10.1038/nrmicro2336 [DOI] [PubMed] [Google Scholar]
- 77.Mouchet J., Airport malaria: a rare disease still poorly understood. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin 5, 75 (2000). [DOI] [PubMed] [Google Scholar]
- 78.Whelan P. et al. , Evidence in Australia for a case of airport dengue. PLoS Negl Trop Dis 6, e1619 (2012). doi: 10.1371/journal.pntd.0001619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuan M.-M., Lin T., Chuang J.-H., Wu H.-S., Epidemiological trends and the effect of airport fever screening on prevention of domestic dengue fever outbreaks in Taiwan, 1998–2007. Int. J. Infect. Dis. 14, e693–e697 (2010). doi: 10.1016/j.ijid.2009.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mohammed H. P. et al. , Travel-associated dengue infections in the United States, 1996 to 2005. J. Travel Med. 17, 8–14. doi: 10.1111/j.1708-8305.2009.00374.x [DOI] [PubMed] [Google Scholar]
- 81.Gautret P. et al. , Infectious diseases among travellers and migrants in Europe, EuroTravNet 2010. Euro Surveillance 17, 20205 (2012). [PubMed] [Google Scholar]
- 82.Warrilow D., Sources of Dengue Viruses Imported into Queensland, Australia, 2002–2010-Volume 18, Number 11—November 2012-Emerging Infectious Disease journal-CDC. (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Warne B. et al. , Travel-related infection in European travelers, EuroTravNet 2011. J. Travel Med. 21, 248–254 (2014). doi: 10.1111/jtm.12120 [DOI] [PubMed] [Google Scholar]
- 84.Cullen K. A., Malaria surveillance—United States, 2013. MMWR. Surveill. Summ. 65, (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
The manuscript relies entirely on data from previously published studies cited in the manuscript. Key estimates derived from the original data are reported in the main text and supporting information.