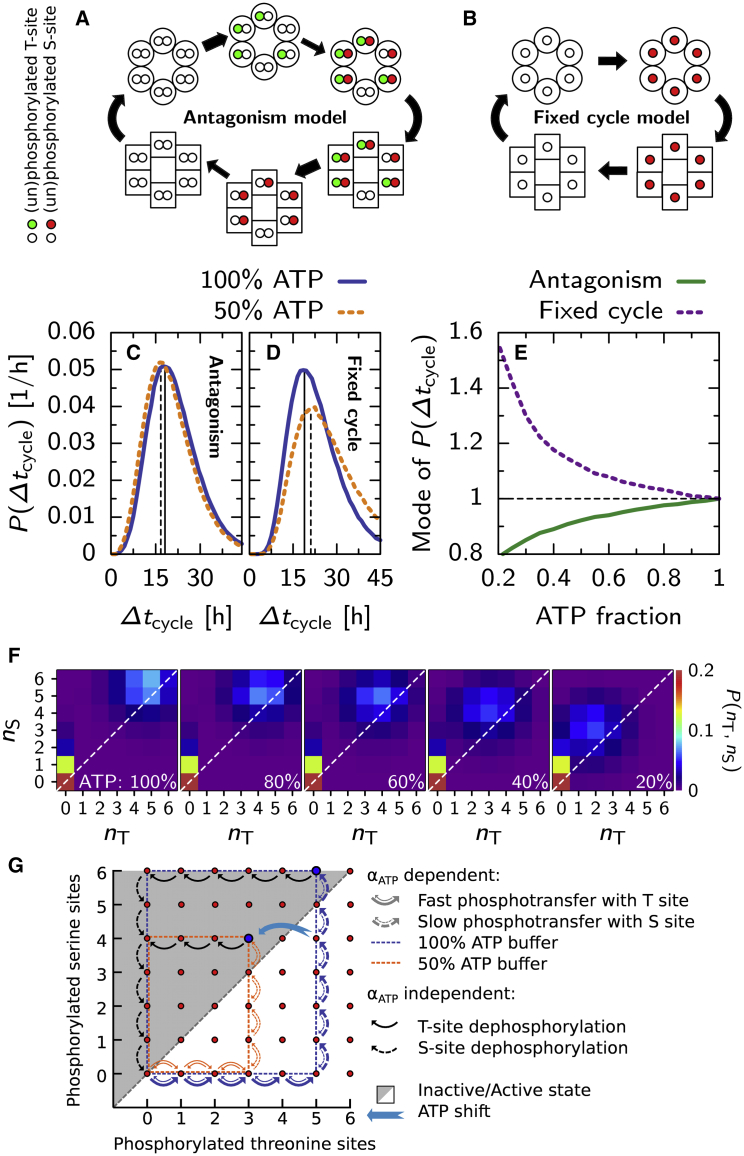
Figure 6.
Antagonistic regulation of the timing of the conformational switch of KaiC creates input compensation in a single hexamer. In the antagonism model (A), fast phosphorylation of the threonine sites stabilizes the active conformation (round monomers), and slow phosphorylation of the serine sites stabilizes the inactive state (square monomers; see key left of (A)). Phosphotransfer rates depend on αATP in the active state, but not in the inactive state. We compare this model with the fixed cycle model (B), where the phosphorylation of a monomer’s single phosphorylation site stabilizes the inactive conformation. (C and D) Given here is the distribution of cycle times, Δtcycle, defined as the time interval between two consecutive switches to the active state, for the antagonism model (C) and the fixed cycle model (D) at 100% (solid line) and 50% (dashed line) ATP buffer. (E) Given here is the mode of the distribution of cycle times for different ATP fractions in the buffer, for the antagonism (solid line), and fixed cycle (dashed line) model. (F) Given here is the distribution in phosphorylation state-space, P(nT, nS), of where the hexamer switches to the inactive state (top-right, along diagonal) and the active state (lower-left corner), at different bulk ATP fractions. As αATP decreases, the hexamer flips to the inactive state at lower phosphorylation levels. (G) Cartoon shows typical cycles through phosphorylation state-space for a hexamer in the antagonism model in a 100% and 50% ATP buffer (see key in (G)). At 100% ATP, the equilibrium level of phosphorylated threonine sites in the active state is higher than at 50% ATP, forcing the hexamer through a larger cycle as it can only change conformation when it is left of the diagonal through the state space (gray area). This compensates for the fact that it progresses through the cycle at a higher rate, such that the time to complete a full cycle is less affected. A temporary lowering of the ATP level from 100 to 50% can induce a phase advance as the phosphorylation level of the threonine sites adjusts to a lower equilibrium level, allowing the hexamer to switch to the inactive state, as indicated by the light-blue arrow. To see this figure in color, go online.