Fig 2.
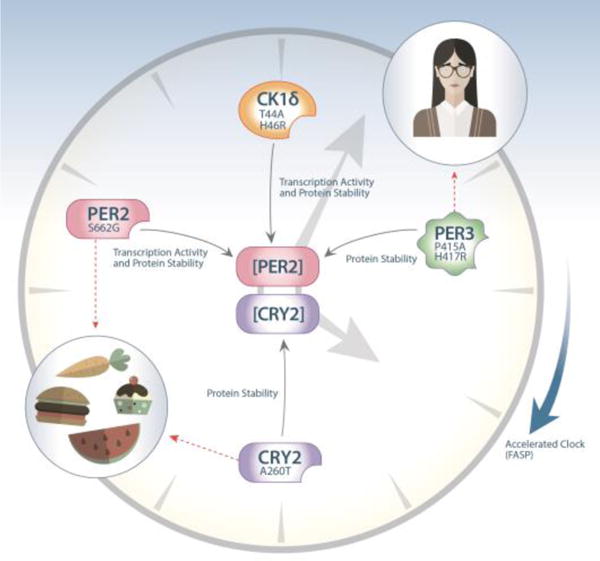
Schematic diagram of the known FASP mutations found in humans. PER2 and CRY2 are core clock components and their protein levels are tightly regulated in a circadian manner to ensure stable clock oscillation. All mutations discovered to date impact the protein levels of these two critical core clock components. The PER2 S662G mutation results in hypophosphorylated of PER2 by casein kinase I (CKI), which in turn causes both increased repressor activity and decreased protein stability. Consistent with this theme, two CK1δ mutations (T44A and H46R) were found to decrease its enzyme activity for substrates including the PER2 S662 site. Thus, these mutations also lead to altered transcription repressor activity and protein stability of PER2. Another FASP mutations, PER3 P415A/H417R, reduces the ability of PER3 to stabilize PER2, resulting in decreased protein stability once again. In addition to this PER2 axis, a more recent finding revealed that the CRY2 A260T mutation increases its accessibility and affinity for ubiquitin E3 ligase, thus promoting its degradation. Both the PER2 and CRY2 mutations have provided insight into the connection between circadian clock and metabolism, while the PER3 mutation offers possible revelation in linking clock and mood regulation. In all the cases mentioned here, the mutations accelerate the endogenous clock, causing mutation carriers to wake up around 4:00 o’clock in the morning. As PERs and CRYs are the major repressive factors in the circadian feedback loop, these genetic findings strongly imply that these repressors play a dominant role in regulating the human sleep wake cycle.
