Figure 3.
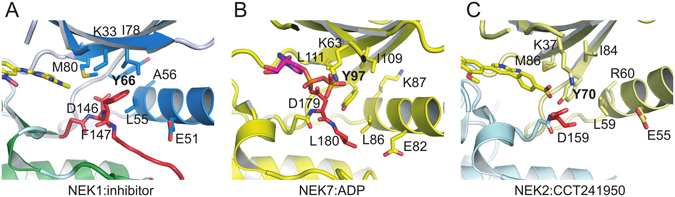
DFG and αC-helix conformations. Tyr66 of NEK1 does not rotate into the binding site as seen for Tyr97 of NEK7 or Tyr70 of NEK2, although all structures are in the inactive state with similar positions of helix αC. (A) View of the DFG motif and αC-helix of NEK1. The DFG motif is in a “DFG-out” inactive conformation, shown in red, and the αC-helix containing residues Leu55, Ala56 and Glu51 is in blue. The salt bridge between Lys33 and Glu51 that would be expected in an active state of NEK1 is absent, helix αC is moved outward, and Lys33 is partly disordered. (B) View from the same angle as A of the DLG motif of NEK7 (equivalent to NEK1 DFG motif) with the DL in red (Gly181 is disordered). Figure based on the structure of NEK7:ADP (PDB ID 2WQN). (C) View from the same angle as A of the DFG motif of NEK2 shown in red (Phe160 and Gly161 are disordered). Figure based on the structure of NEK2:CCT241950 (PDB ID 2WQO).
