Figure 5.
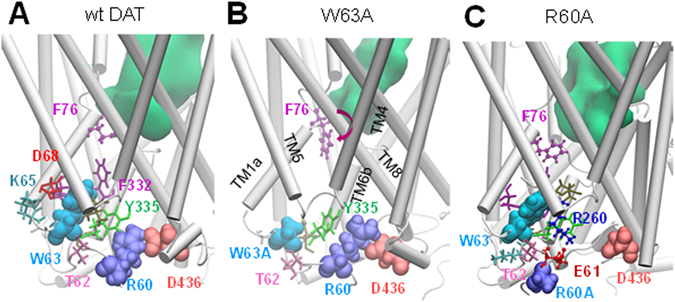
The IC interaction network that regulates the opening/closure of the IC vestibule and the transition between OF and IF states, shows distinctive dynamics in wt DAT and mutants W63A and R60A. (A) In wt DAT, the salt-bridge R60-D436 and close interactions between W63 and the F332 and Y335 stabilize substrate/sodium-binding site in the OF state. (B) In the mutant W63A, substitution of W63 by alanine breaks the stabilizing network of molecular interaction; F76 side chain dihedral angle χ 1 rotates from ± 180° to −60°, leading to the opening of the IC vestibule. (C) Substitution of R60 by alanine breaks the salt-bridge R60-D436. Yet, alternative IC salt bridges form, such as R260-E61, which stably maintain the closed state of the IC vestibule. Residues at positions 60 and 63 are displayed in VDW format, as well as D436. In all diagrams, residues within 3 Å of W63 or W63A are shown in licorice format. Hydrated EC regions are indicated by green shaded areas.
