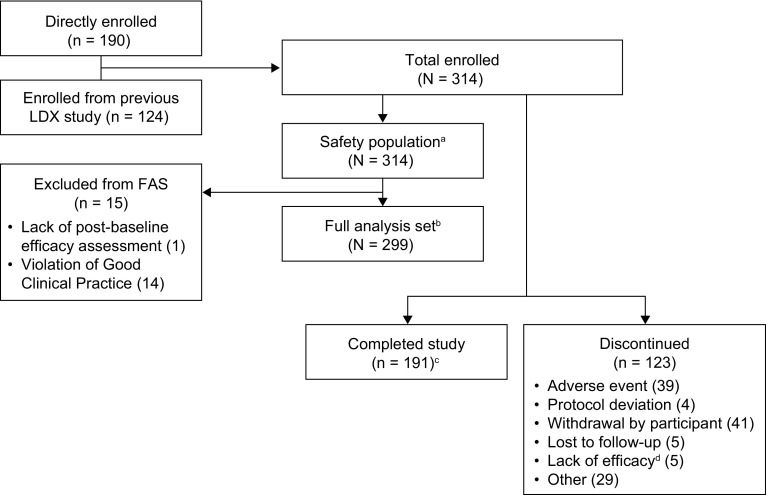
Fig. 2.
Patient disposition. aThe safety population comprised all enrolled participants who received at least one dose of LDX during the study. bThe FAS comprised all participants who received one dose of LDX and had at least one on-treatment post-baseline efficacy assessment; all 14 participants from a single study site were excluded from the efficacy analyses because of a serious violation of Good Clinical Practice. cThe number of participants refers to individuals in the enrolled population who completed the study. A total of 191 participants who were included in the FAS completed the study. dAccording to the protocol, lack of efficacy (in the opinion of the investigator) was to be reported as an adverse event. Five additional patients discontinued because of investigator-perceived lack of efficacy; according to the protocol, these should have been recorded as treatment-emergent adverse events. FAS full analysis set, LDX lisdexamfetamine dimesylate