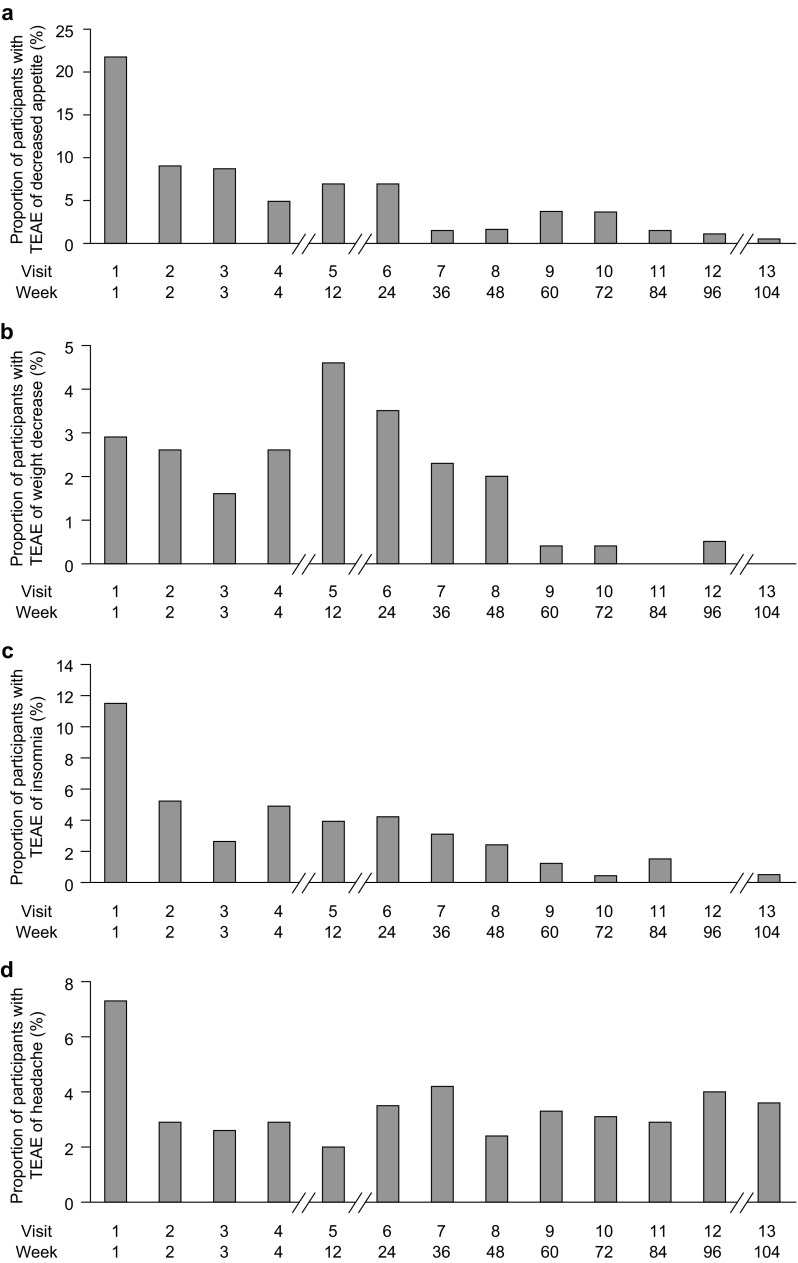
Fig. 3.
Incidence of treatment-emergent adverse events identified by the sponsor as of special interest: a decreased appetite, b weight decrease, c insomnia, and d headache (safety population). Percentages are based on the number of participants in the safety population who received lisdexamfetamine dimesylate for the given week. TEAE treatment-emergent adverse event