Abstract
Serological tests used in current brucellosis eradication schemes, such as bacterial tube agglutination, do not readily distinguish between infected animals and those immunized with strain 19 or 45/20 Brucella abortus vaccines. In this study, sera from naturally infected cattle were used to identify serologically important antigens in extracts of virulent B. abortus by gel diffusion techniques. Antisera from rabbits hyperimmunized with selected precipitation lines were used for purification by affinity chromatography of two precipitating and one non-precipitating antigen from crude bacterial extracts. A passive hemagglutination test using these antigens was developed. A number of characterized bovine sera were screened by passive hemagglutination and conventional bacterial tube agglutination test. A considerable improvement in discrimination between sera from infected and vaccinated cattle was obtained with the hemagglutination test compared with bacterial tube agglutination.
Full text
PDF

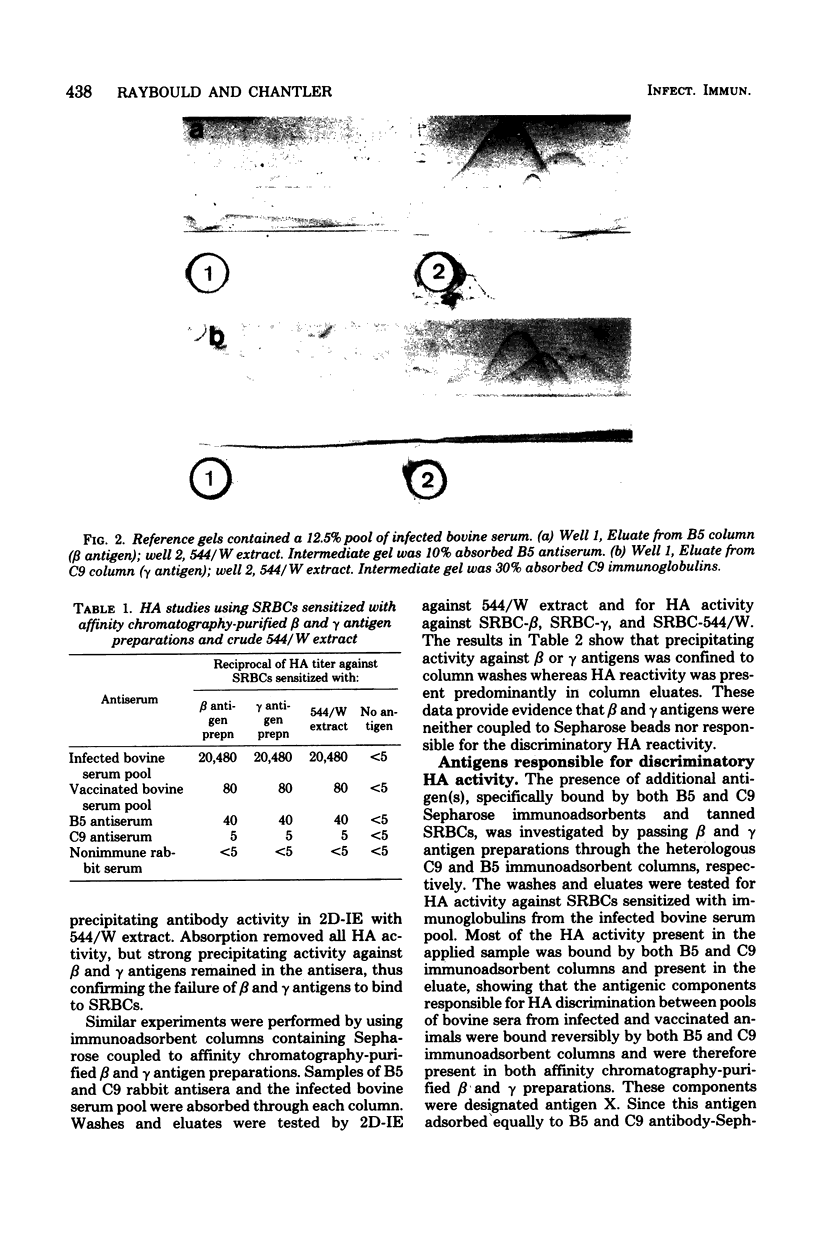


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan G. S., Chappel R. J., Williamson P., McNaught D. J. A quantitative comparison of the sensitivity of serological test for bovine brucellosis to different antibody classes. J Hyg (Lond) 1976 Apr;76(2):287–298. doi: 10.1017/s0022172400055182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alton G. G. Recent developments in vaccination against bovine brucellosis. Aust Vet J. 1978 Dec;54(12):551–557. doi: 10.1111/j.1751-0813.1978.tb02410.x. [DOI] [PubMed] [Google Scholar]
- Beh K. J. Quantitative distribution of Brucella antibody amongst immunoglobulin classes in vaccinated and infected cattle. Res Vet Sci. 1974 Jul;17(1):1–4. [PubMed] [Google Scholar]
- Chappel R. J., McNaught D. J., Bourke J. A., Allan G. S. Comparison of the results of some serological tests for bovine brucellosis. J Hyg (Lond) 1978 Jun;80(3):365–371. doi: 10.1017/s0022172400024815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappel R. J., McNaught D. J., Bourke J. A., Allan G. S. The diagnostic efficiency of some serological tests for bovine brucellosis. J Hyg (Lond) 1978 Jun;80(3):373–384. doi: 10.1017/s0022172400024827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton M. J., Parkhouse R. M.E. Comparison of the effects of various detergents on antigen-antibody interaction. FEBS Lett. 1972 May 1;22(2):210–212. doi: 10.1016/0014-5793(72)80047-4. [DOI] [PubMed] [Google Scholar]
- Diaz R., Jones L. M., Leong D., Wilson J. B. Surface antigens of smooth brucellae. J Bacteriol. 1968 Oct;96(4):893–901. doi: 10.1128/jb.96.4.893-901.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis G. J. Effect of detergents on antibody-antigen interaction. Anal Biochem. 1979 Oct 1;98(2):445–451. doi: 10.1016/0003-2697(79)90165-9. [DOI] [PubMed] [Google Scholar]
- Freeman B. A., Baughn R. E., McGhee J. R. Some physical, chemical, and taxonomic features of the soluble antigens of the Brucellae. J Infect Dis. 1970 May;121(5):522–527. doi: 10.1093/infdis/121.5.522. [DOI] [PubMed] [Google Scholar]
- Hinsdill R. D., Berman D. T. Antigens of Brucella abortus. I. Chemical and immunoelectrophoretic characterization. J Bacteriol. 1967 Feb;93(2):544–549. doi: 10.1128/jb.93.2.544-549.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannelli D., Diaz R., Bettini T. M. Identification of Brucella abortus antibodies in cattle serum by single radial diffusion. J Clin Microbiol. 1976 Feb;3(2):203–205. doi: 10.1128/jcm.3.2.203-205.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince A. M., Ikram H., Chicot D., Wright R., Vnek J., Neurath R., Lippin A., Swiss S. A new reversed passive hemagglutination test for detection of HBsAg. Vox Sang. 1975;29(5):319–329. doi: 10.1111/j.1423-0410.1975.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Qualtiere L. F., Anderson A. G., Meyers P. Effects of ionic and nonionic detergents on antigen-antibody reactions. J Immunol. 1977 Nov;119(5):1645–1651. [PubMed] [Google Scholar]
- Raybould T. J., Chantler S. Comparison of activation and blocking procedures in the use of defined antigen substrate spheres (DASS) for quantitative and visual assessment of conjugate activity. J Immunol Methods. 1979;27(4):309–318. doi: 10.1016/0022-1759(79)90208-4. [DOI] [PubMed] [Google Scholar]
- Raybould T. J., Chantler S. Serological differentiation between infected and vaccinated cattle by visual and quantitative immunofluorescence using Brucella abortus antigen coupled sepharose beads. J Immunol Methods. 1979;30(1):37–46. doi: 10.1016/0022-1759(79)90271-0. [DOI] [PubMed] [Google Scholar]
- Rice C. E., Cochrane D., Tailyour J. Electrophoretic studies of sera from cattle vaccinated or naturally infected with Brucella abortus. Can J Comp Med Vet Sci. 1966 Jun;30(6):161–168. [PMC free article] [PubMed] [Google Scholar]
- Rice C. E., Tailyour J., Cochrane D. Ultracentrifugal studies of sera from cattle vaccinated or naturally infected with Brucella abortus. Can J Comp Med Vet Sci. 1966 Oct;30(10):270–278. [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J., Wilson D. V. A simple method for coupling proteins to insoluble polysaccharides. Immunology. 1971 Jun;20(6):1061–1065. [PMC free article] [PubMed] [Google Scholar]
- Schurig G. G., Jones L. M., Speth S. L., Berman D. T. Antibody response to antigens distinct from smooth lipopolysaccharide complex in Brucella infection. Infect Immun. 1978 Sep;21(3):994–1002. doi: 10.1128/iai.21.3.994-1002.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemshorn B., Nielsen K. The bovine immune response to Brucella abortus I. A water soluble antigen precipitated by sera of some naturally infected cattle. Can J Comp Med. 1977 Apr;41(2):152–159. [PMC free article] [PubMed] [Google Scholar]
- Weeke B. Crossed immunoelectrophoresis. Scand J Immunol Suppl. 1973;1:47–56. doi: 10.1111/j.1365-3083.1973.tb03778.x. [DOI] [PubMed] [Google Scholar]
- Wood W. A., Corbel M. J. Concentrations of bovine serum protein classes in relation to reactivity in serological tests for brucellosis. J Comp Pathol. 1973 Jan;83(1):143–150. doi: 10.1016/0021-9975(73)90037-6. [DOI] [PubMed] [Google Scholar]