Abstract
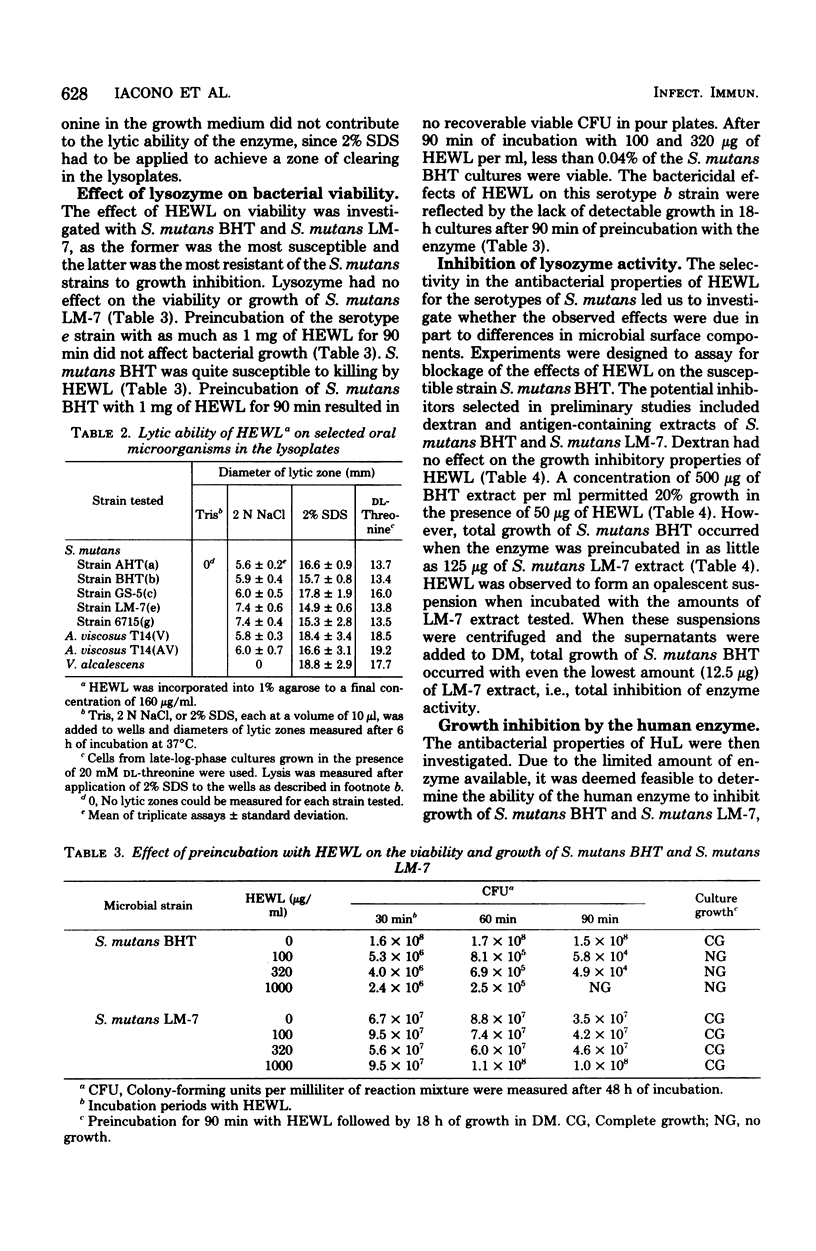
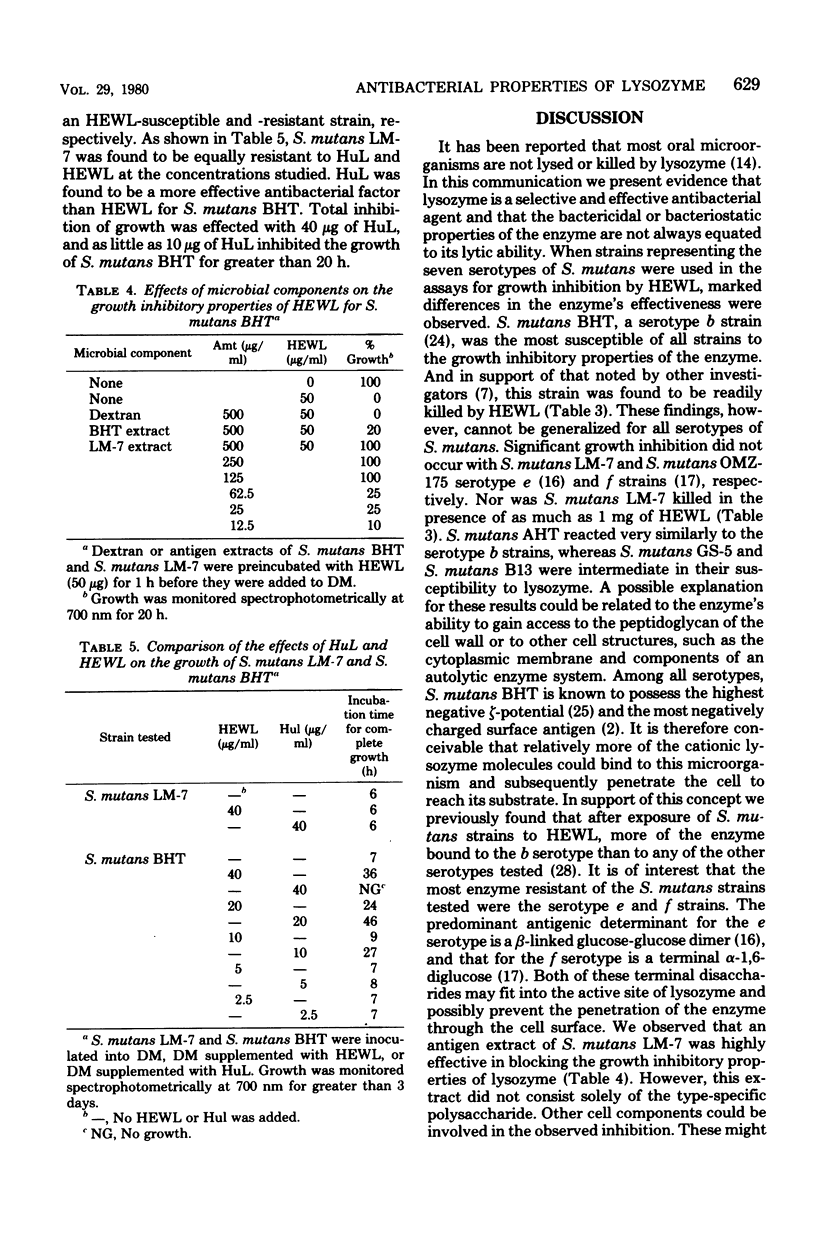
The antibacterial properties of lysozyme were investigated with oral microorganisms representing the seven serotypes (a through g) of Streptococcus mutans, Veillonella alcalescens, and the virulent (V) and avirulent (AV) strains of Actinomyces viscosus T14. Growth of bacteria in defined medium was monitored spectrophotometrically after the addition of various amounts (25 μg to 5 mg/ml) of enzyme. No growth inhibition of V. alcalescens was observed. Inhibition of A. viscosus T14(V) and A. viscosus T14(AV) occurred with 160 μg of lysozyme per ml. Of the S. mutans cultures tested, the serotype a and b strains were inhibited with as little as 25 μg of enzyme per ml, whereas e and f strains were most resistant to the bacteriostatic activity of lysozyme. The presence of dl-threonine or sucrose in growth medium did not significantly affect the results. A lysoplate assay was developed to rapidly survey the bacterial cultures for their susceptibility to the lytic ability of the enzyme. Lysis, as a measure of a zone of clearing in agarose plates, occurred for all microorganisms in the presence of lysozyme after the subsequent addition of NaCl or detergent. The bactericidal activity of lysozyme was determined on S. mutans BHT and S. mutans LM-7 by the pour plate technique. Preincubation of S. mutans LM-7 with as much as 1 mg of enzyme for 90 min did not affect viability or growth, whereas preincubation of S. mutans BHT with 1 mg of lysozyme resulted in no recoverable colony-forming units. An antigen containing extract of S. mutans LM-7 blocked the growth inhibitory property of lysozyme. Human lysozyme was a more effective antibacterial factor than hen egg white lysozyme. Total growth inhibition of S. mutans BHT was effected with 40 μg of human enzyme, and as little as 10 μg of human enzyme inhibited growth for greater than 20 h. The data presented indicate that different mechanisms may be responsible for the bacteriostatic, lytic, and bactericidal properties of the enzyme and that lysozyme is a selective but effective antibacterial factor for oral microorganisms.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLADEN H. A., MERGENHAGEN S. E. ULTRASTRUCTURE OF VEILLONELLA AND MORPHOLOGICAL CORRELATION OF AN OUTER MEMBRANE WITH PARTICLES ASSOCIATED WITH ENDOTOXIC ACTIVITY. J Bacteriol. 1964 Nov;88:1482–1492. doi: 10.1128/jb.88.5.1482-1492.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Carroll S. F., Martinez R. J. Role of rabbit lysozyme in in vitro serum and plasma serum bactericidal reactions against Bacillus subtilis. Infect Immun. 1979 Sep;25(3):810–819. doi: 10.1128/iai.25.3.810-819.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M. A gentle method for the lysis of oral streptococci. Biochem Biophys Res Commun. 1976 Jan 26;68(2):603–608. doi: 10.1016/0006-291x(76)91188-8. [DOI] [PubMed] [Google Scholar]
- Coleman S. E., Van de Rijn I., Bleiweis A. S. Lysis of cariogenic and noncariogenic oral streptococci with lysozyme. J Dent Res. 1971 Jul-Aug;50(4):939–943. doi: 10.1177/00220345710500042601. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. C., Neuberger A. Modification of lysine and arginine residues of lysozyme and the effect on enzymatic activity. Biochim Biophys Acta. 1969 Apr 22;178(2):306–317. doi: 10.1016/0005-2744(69)90398-2. [DOI] [PubMed] [Google Scholar]
- Day D. F., Marceau-Day M. L., Ingram J. M. Protein-lipopolysaccharide interactions. 1. The reaction of lysozyme with Pseudomonas aeruginosa LPS. Can J Microbiol. 1978 Feb;24(2):196–199. doi: 10.1139/m78-035. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. J., Lillmars K. A method for gentle lysis of Streptococcus sanguis and Streptococcus mutans. Biochem Biophys Res Commun. 1975 Jul 8;65(1):378–384. doi: 10.1016/s0006-291x(75)80104-5. [DOI] [PubMed] [Google Scholar]
- GLADSTONE G. P., JOHNSTON H. H. The effect of cultural conditions on the susceptibility of Bacillus anthracis to lysozyme. Br J Exp Pathol. 1955 Aug;36(4):363–372. [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., de Stoppelaar J. D., Harden L. Lysozyme insensitivity of bacteria indigenous to the oral cavity of man. J Dent Res. 1966 May-Jun;45(3):877–881. doi: 10.1177/00220345660450036201. [DOI] [PubMed] [Google Scholar]
- Hamada S., Gill K., Slade H. D. Chemical and immunological properties of the type f polysaccharide antigen of Streptococcus mutans. Infect Immun. 1976 Jul;14(1):203–211. doi: 10.1128/iai.14.1.203-211.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Purification and immunochemical characterization of type e polysaccharide antigen of Streptococcus mutans. Infect Immun. 1976 Jul;14(1):68–76. doi: 10.1128/iai.14.1.68-76.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Torii M., Kotani S., Masuda N., Ooshima T., Yokogawa K., Kawata S. Lysis of Streptococcus mutans cells with mutanolysin, a lytic enzyme prepared from a culture liquor of Streptomyces globisporus 1829. Arch Oral Biol. 1978;23(7):543–549. doi: 10.1016/0003-9969(78)90268-6. [DOI] [PubMed] [Google Scholar]
- Holden J. T., Grant W., Degroot J. Lysozyme sensitivity of pantothenate-deficient Lactobacillus plantarum grown with exogenous fatty acids. Biochim Biophys Acta. 1979 Jul 27;574(1):173–176. doi: 10.1016/0005-2760(79)90096-1. [DOI] [PubMed] [Google Scholar]
- Inoue M., Hamada S., Ooshima T., Kotani S., Kato K. Chemical composition of Streptococcus mutans cell walls and their susceptibility to Flavobacterium L-11 enzyme. Microbiol Immunol. 1979;23(5):319–328. doi: 10.1111/j.1348-0421.1979.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y., Watanabe T., Tsunemitsu A., Fukui Z., Moriyama T. Lysis of streptococci by lysozyme from human parotid saliva and sodium lauryl sulfate. J Dent Res. 1971 Nov-Dec;50(6):1688–1688. doi: 10.1177/00220345710500066201. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Chemical composition and immunological specificity of the streptococcal group O cell wall polysaccharide antigen. Infect Immun. 1972 May;5(5):707–714. doi: 10.1128/iai.5.5.707-714.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Structure and immunological specificity of the Streptococcus mutans group b cell wall antigen. Infect Immun. 1973 Apr;7(4):578–585. doi: 10.1128/iai.7.4.578-585.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson J., Glantz P. O., Krasse B. Electrophoretic mobility of oral streptococci. Arch Oral Biol. 1976;21(10):605–609. doi: 10.1016/0003-9969(76)90030-3. [DOI] [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRASAD A. L., LITWACK G. MEASUREMENT OF THE LYTIC ACTIVITY OF LYSOZYMES (MURAMIDASES). Anal Biochem. 1963 Oct;6:328–334. doi: 10.1016/0003-2697(63)90157-x. [DOI] [PubMed] [Google Scholar]
- Perch B., Kjems E., Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. The anatomy of the bacterial surface. Bacteriol Rev. 1961 Jun;25:77–99. doi: 10.1128/br.25.2.77-99.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOPHIANOPOULOS A. J., RHODES C. K., HOLCOMB D. N., VAN HOLDE K. E. Physical studies of lysozyme. I. Characterization. J Biol Chem. 1962 Apr;237:1107–1112. [PubMed] [Google Scholar]
- Saint-Blancard J., Chuzel P., Mathieu Y., Perrot J., Jollès P. Influence of pH and ionic strength of the lysis of Micrococcus lysodeikticus cells by six human and four avian lysozymes. Biochim Biophys Acta. 1970 Nov 11;220(2):300–306. doi: 10.1016/0005-2744(70)90014-8. [DOI] [PubMed] [Google Scholar]
- Selsted M. E., Martinez R. J. Lysozyme: primary bactericidin in human plasma serum active against Bacillus subtilis. Infect Immun. 1978 Jun;20(3):782–791. doi: 10.1128/iai.20.3.782-791.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Cornett J. B., Mychajlonka M. Does penicillin kill bacteria?. Rev Infect Dis. 1979 Sep-Oct;1(5):787–796. doi: 10.1093/clinids/1.5.787. [DOI] [PubMed] [Google Scholar]
- Taichman N. S., Hammond B. F., Tsai C. C., Baehni P. C., McArthur W. P. Interaction of inflammatory cells and oral microorganisms. VII. In vitro polymorphonuclear responses to viable bacteria and to subcellular components of avirulent and virulent strains of Actinomyces viscosus. Infect Immun. 1978 Aug;21(2):594–604. doi: 10.1128/iai.21.2.594-604.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witholt B., Boekhout M. The effect of osmotic shock on the accessibility of the murein layer of exponentially growing Escherichia coli to lysozyme. Biochim Biophys Acta. 1978 Apr 4;508(2):296–305. doi: 10.1016/0005-2736(78)90332-2. [DOI] [PubMed] [Google Scholar]
- de Boer W. R., Kruyssen F. J., Wouters J. T. Cell wall metabolism of Bacillus subtilis [proceedings]. Antonie Van Leeuwenhoek. 1979;45(2):315–317. doi: 10.1007/BF00418596. [DOI] [PubMed] [Google Scholar]