Neurons of the central complex in several insects signal compass directions through sensitivity to the sky polarization pattern. In locusts, these neurons also respond to moving objects. We show here that during polarized-light presentation, responses to moving objects override their compass signaling or restore adapted inhibitory as well as excitatory compass responses. A network model is presented to explain the variations of these responses that likely serve to redirect flight or walking following evasive maneuvers.
Keywords: insect brain, central complex, desert locust, spatial orientation, sky compass, escape behavior, goal conflict, gain modulation, context dependency, neural modeling
Abstract
Goal-directed behavior is often complicated by unpredictable events, such as the appearance of a predator during directed locomotion. This situation requires adaptive responses like evasive maneuvers followed by subsequent reorientation and course correction. Here we study the possible neural underpinnings of such a situation in an insect, the desert locust. As in other insects, its sense of spatial orientation strongly relies on the central complex, a group of midline brain neuropils. The central complex houses sky compass cells that signal the polarization plane of skylight and thus indicate the animal’s steering direction relative to the sun. Most of these cells additionally respond to small moving objects that drive fast sensory-motor circuits for escape. Here we investigate how the presentation of a moving object influences activity of the neurons during compass signaling. Cells responded in one of two ways: in some neurons, responses to the moving object were simply added to the compass response that had adapted during continuous stimulation by stationary polarized light. By contrast, other neurons disadapted, i.e., regained their full compass response to polarized light, when a moving object was presented. We propose that the latter case could help to prepare for reorientation of the animal after escape. A neuronal network based on central-complex architecture can explain both responses by slight changes in the dynamics and amplitudes of adaptation to polarized light in CL columnar input neurons of the system.
NEW & NOTEWORTHY Neurons of the central complex in several insects signal compass directions through sensitivity to the sky polarization pattern. In locusts, these neurons also respond to moving objects. We show here that during polarized-light presentation, responses to moving objects override their compass signaling or restore adapted inhibitory as well as excitatory compass responses. A network model is presented to explain the variations of these responses that likely serve to redirect flight or walking following evasive maneuvers.
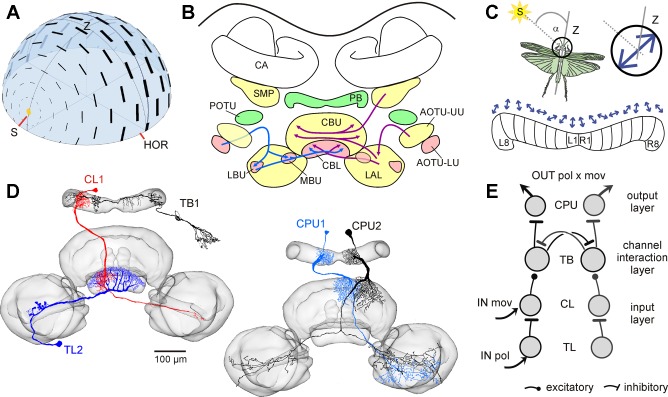
a pattern of polarization, produced by scattering of sunlight in the blue sky (Fig. 1A) and invisible to the human eye, is used by many insects for spatial orientation (Wehner 1976; Wehner and Labhart 2006). Photoreceptors in a specialized dorsal eye region, the dorsal rim area, are sensitive to the plane of polarized light (Labhart and Meyer 1999). Their signals are transmitted through several stages of processing in the optic lobe and anterior optic tubercle and finally converge from both brain hemispheres in the central complex, a group of midline brain structures including the protocerebral bridge and the central body (Homberg et al. 2011; Mota et al. 2011; Pfeiffer and Kinoshita 2012; Fig. 1B). Evidence from the desert locust, field cricket, monarch butterfly, and two species of dung beetle suggests that the central complex houses a network of neurons signaling compass directions based on the celestial polarization pattern (el Jundi et al. 2015; Heinze and Homberg 2007; Heinze and Reppert 2011; Sakura et al. 2008). In desert locusts, polarized-light signals enter the central complex via tangential neurons of the lower division of the central body (TL neurons). Signals are transmitted via intermediate-stage columnar neurons of the lower division of the central body (CL neurons) to tangential neurons of the protocerebral bridge (TB neurons) and finally to columnar neurons of the protocerebral bridge and upper division of the central body (CPU1 and CPU2 neurons) representing the output stage of processing in the central complex (Fig. 1, D and E; Bockhorst and Homberg 2015a; Heinze et al. 2009). CPU neurons provide a compass-like representation of electric field vectors (E-vectors) of polarized light based on their ramifications in single slices of the protocerebral bridge (Fig. 1C). They are thus suited to signal sun-compass directions based on an activity peak in a particular slice. When stimulated from above with light passing through a rotating polarizer, neurons were either excited at a particular angular orientation of the polarizer (TL neurons), inhibited at a particular orientation of the polarizer (CL neurons), or showed maximum excitation and inhibition at orthogonal orientations of the polarizer, respectively (preferred and antipreferred orientation; TB and CPU cells) (Bockhorst and Homberg 2015a). When stimulated through a stationary polarizer, TL2 neurons showed tonic activity levels corresponding to the orientation of the polarizer relative to their preferred E-vector orientation, but all types of neuron downstream to TL2 showed rapid adaptation (Bockhorst and Homberg 2015a).
Fig. 1.
Neural substrates of sky-compass sensing in the locust brain. A: polarization pattern of the blue sky. Electric field (E)-vectors (black bars) are arranged tangentially along concentric circles around the sun. The degree of polarization (thickness of bars) is maximal at an angle of 90° from the sun (S). HOR, horizon; Z, zenith. B: visual pathways to the central complex. The polarization-vision pathway to the central complex is shown on the left (red neuropils, blue arrows), and the pathways that might signal events in the visual scenery are on the right (yellow neuropils, magenta arrows). AOTU-UU, AOTU-LU, upper, resp. lower unit of the anterior optic tubercle; CA, calyx of the mushroom body; CBL, CBU, lower, resp. upper division of the central body; LAL, lateral accessory lobe; LBU, MBU, lateral, resp. medial bulb; PB, protocerebral bridge; POTU, posterior optic tubercle; SMP, superior medial protocerebrum. C: the robust relationship between E-vector pattern and solar position may serve to align the direction of locomotion (α) relative to the sun. Preferred E-vector angles (blue arrows) of output cells in the central-complex network vary systematically along the slices of the PB, spanning a range of 360° across the 16 vertical slices (L1-L8, R1-R8). D and E: major types of polarization-sensitive neuron of the central complex (D, frontal views) and their putative wiring pattern (E), based on data from Bockhorst and Homberg (2015a, 2015b). Tangential neurons of the CBL (TL neurons) invade entire layers of the CBL. Tangential TB neurons invade slices within the PB and layers in the POTU. Columnar neurons connect distinct slices of the PB to the CBU (CPU neurons) or CBL (CL neurons) and have additional arborizations in the lateral accessory lobe. CPU neurons are the principal output elements of the network. A is courtesy of Dr. Keram Pfeiffer, illustrated similarly by Rossel and Wehner (1987); B is modified from Pfeiffer and Homberg (2014); and D is modified from el Jundi et al. (2010), Heinze and Homberg (2007), and Bockhorst and Homberg (2015b).
Recent experiments revealed that neurons of the locust sky-compass network in the central complex also respond to expanding black disks (looming stimuli) and small moving objects presented laterally (Bockhorst and Homberg 2015b; Rosner and Homberg 2013). In the absence of polarized light, CL, TB, and CPU neurons responded to moving objects irrespective of moving trajectory and direction, but only if the object was novel, i.e., they responded only to the first in a series of identical stimuli (Bockhorst and Homberg 2015b; Rosner and Homberg 2013). The responses to repetitive looming stimuli appeared to be more robust, but in those experiments longer intervals were allowed between stimuli (Rosner and Homberg 2013). To study the interaction of sensitivities to moving objects and polarized light we tested in the present study the responses of central-complex neurons to moving objects in the lateral field of view during concurrent presentation of polarized light through a stationary polarizer above the animal.
MATERIALS AND METHODS
Experimental animals, intracellular recordings, and histology.
Desert locusts (Schistocerca gregaria) were reared in crowded indoor colonies under an 11:13-h light-dark regime at 28°C. Males were preferred over females for lower content of fat in hemolymph, which eases preparation. Details on preparation, intracellular recordings, and histological processing are provided in Bockhorst and Homberg (2015a). After removal of antennae, wings, and legs, the frontal brain surface was accessed via an excision of the frontal cuticle of the head. To stabilize recordings, muscles in the vicinity of the brain were transected, a spoon-shaped wire loop was used to support the brain from posterior, and the gut was removed to stop peristaltic pumping. Electrode insertion was facilitated by an incision of the neural sheath of the brain. During preparation and recording, the brain was kept immersed in locust saline (Clements and May 1974). Each recording was obtained from a separate animal.
Sharp micropipettes (50-200 MΩ) filled with 1 M KCl were used for intracellular recording and cell labeling, and their tips were loaded with Neurobiotin tracer (Vector Laboratories, Burlingame, UK; 4% in 1 M KCl) for iontophoretic injection (0.5–2 nA, 1–15 min) after recording. Connectors and reference electrodes immersed in the saline outside the brain were made from Ag-AgCl wire. Digitized signals were analyzed offline using software written in MATLAB (MathWorks, Natick, MA). Intracellular recordings lasted for 10 to 45 min.
For visualization of neural morphologies, whole mount preparations were fixed chemically and incubated in a solution of streptavidin-conjugated Cy3 fluorophore (Dianova, Hamburg, Germany) that targets the Neurobiotin tracer. Subsequently, brains were dehydrated in an ethanol series, cleared using methyl salicylate, embedded in Permount (Fisher Scientific, Pittsburgh, PA), and scanned confocally (Leica TCS SP5 confocal laser scanning microscope; Leica Microsystems, Wetzlar, Germany) at either ×10 or ×20 magnification. Cy3-fluorescence was induced by excitation at 561 nm (DPSS laser). Relevant neuropils were identified based on their autofluorescence.
Visual stimulation.
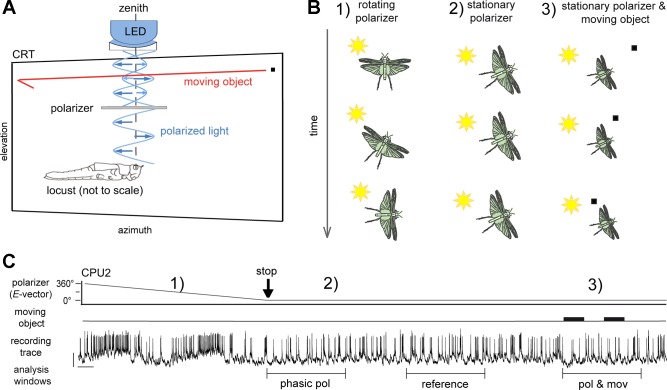
Compass stimulation was applied by means of blue light (range: 421.6–524.3 nm; peak: 461.11 nm; and 1015 photons⋅cm−2⋅s−1) from an LED source (ELJ-465–617; EPIGAP Optoelektronik, Berlin, Germany), passing a rotatable linear polarizer (HN38S; Polaroid, Cambridge, MA; 20-mm inner diameter) positioned zenithal to the head at 60-mm distance (visual angle 19°; Fig. 2A). Neuronal activity was recorded under dim, unilateral wide-field illumination (4.3 × 1013 photons⋅cm−2⋅s−1) by a cathode-ray tube display (Mitsubishi DP2070SB 22”; Mitsubishi, Tokyo, Japan) that was used for the presentation of moving virtual objects. Photon flux was measured with a digital spectrometer (USB2000; Ocean Optics). The display was positioned slightly tilted in the left latero-frontal visual field, covering −45° to +60° in azimuth and −32° to +28° in elevation (Fig. 2A). Virtual objects were generated by MATLAB software (based on the CRS toolbox provided by Cambridge Research Systems, Rochester, Kent, UK) and displayed against the abovementioned dim (“grey”) background using a ViSaGe stimulus device (Cambridge Research Systems).
Fig. 2.
Experimental procedure and data analysis. A: experimental setup for presentation of virtual moving objects and polarized light during intracellular recording. For compass stimulation, blue light emitted from an LED in the zenith was directed through a rotatable linear polarizer onto the locust’s eyes. Blue arrows indicate electric field vectors. A virtual object (black square) in translatory motion (red arrow) was presented on a cathode ray tube display (CRT) in the left antero-lateral visual field. B: the stimulus paradigm included 3 scenarios in succession: 1) rotation of the polarizer, which mimics yaw-movements for alignment relative to the sun, was used to characterize neuronal tuning to E-vectors; 2) stimulation by stationary polarizer as occurring during constant alignment of the body axis relative to the sun; and 3) the combination of scenario 2 with presentations of a moving object. This combined stimulation was applied after responses to the stationary polarizer (scenario 2) had declined to a tonic plateau. C: for evaluation of spiking activity, different analysis windows (5-s duration) were set to capture the phasic response to the stationary E-vector angle (phasic pol), its tonic plateau level (reference), and the response to combined stimulation (pol and mov), respectively. Black vertical arrow (stop) indicates the stop of polarizer rotation and thus the beginning of scenario 2 (stimulation with a stationary E-vector); the 2 thick horizontal bars indicate occurrence of the moving object stimulus. Spiking activity is an example trace recorded from a CPU2 cell. Bars = 10 mV, 1 s.
Moving-object stimuli were based on sequential translations (70°/s) of a single black, rectangular small-field patch (~2° visual angle azimuthal extent and 1.5° elevational extent) in either forward or backward direction along a horizontal trajectory, with a 500- to 1,500-ms pause between translations (Fig. 2A). The trajectory spanned the entire width of the display (−45° to +60° in azimuth) at either high elevation (+25.5°) or low elevation (−29.5°). As the responses appeared to occur independently from direction of motion and trajectory elevation (Bockhorst and Homberg 2015b), data were pooled across stimulus batteries that differed in these respects. Experiments began with measurements of tuning to E-vector angle by means of clockwise and counterclockwise rotations of the polarizer (scenario 1 in Fig. 2, B and C). Subsequent to tuning measurement, the polarizer was rotated to orientations that elicited prominent inhibitory and excitatory responses, respectively (scenario 2 in Fig. 2, B and C). Details on these procedures were described earlier (Bockhorst and Homberg 2015a, 2015b). Once the response to the stationary E-vector had declined, moving objects were presented concurrent with the ongoing presentation of polarized light (scenario 3 in Fig. 2, B and C).
Data evaluation.
Physiological data were accepted for further evaluation if the recorded cell was identified unambiguously. Ideally, this was provided by its distinct labeling. In case of more than one neuron being labeled in the same specimen, measurements were assigned to morphologies by means of cell-type specific features of ongoing activity (Bockhorst and Homberg 2015a). In final analyses, solely those experiments were included that yielded at least 10 measurements of 5-s peristimulus activity during concurrent presentation of compass and object stimuli and measurements at both excitatory and inhibitory E-vector angles within the same experiment.
Spike times were determined via threshold-based event detection, and false positives were excluded by an upper threshold for spike amplitude as well as an absolute refractory period of 1 ms. Rasterplots were generated using a MATLAB function (rasterplot.m) kindly provided by Rajiv Narayan (Boston University), available at http://www.mathworks.com/matlabcentral/fileexchange/10000-rasterplot?requestedDomain=www.mathworks.com. To estimate the instantaneous firing rate for plots of individual recordings, we generated Gaussian-smoothed peristimulus time histograms using the code provided by Kreuz et al. (2011). To quantify phasic responses to polarized light as well as responses to combined stimulation, we compared spike rates in peristimulus time windows to a sample of the adapted response to polarized light alone (Fig. 2C). At this, normalized changes in spike rate were obtained via division of the respective change in spike rate by the spike rate in the reference sample. In few cases in which combined stimulation was started earlier, reference windows shorter than 5 s or located subsequent to combined stimulation were tolerated.
Network modeling.
A neural network was modeled based on MATLAB-code for leaky integrate-and-fire neurons provided as companion material of Gabbiani and Cox (2010) at http://booksite.elsevier.com/9780123748829/. Parameters for synaptic transmission were adjusted as to allow 1:1 transmission of spikes at excitatory CL-TB synapses and 1:1 extinction of spikes by coincident inhibitory postsynaptic potentials at the TB-TB and TB-CPU synapses. Maximum spontaneous activity was set to 15, 30, and 40 impulses/s firing for CL, TB, and CPU respectively, with minimal interstimulus interval jitter. Mean rates were set to values typical for these types of neuron (Bockhorst and Homberg 2015a). A refractory period of 3 ms was applied in the integrate-and-fire processes.
RESULTS
The data are based on 16 recordings from neurons of the locust central complex, including four TL2 neurons, three CL1 neurons, five TB1 neurons, two CPU1 neurons, and two CPU2 neurons. Neurons were first tested for responses to polarized light alone by light passing through a dorsally rotating polarizer. We then stopped rotation of the polarizer at an E-vector that produced an excitatory response (preferred orientation; TL, TB, and CPU cells), an inhibitory one (antipreferred orientation; CL, TB, and CPU cells), or no apparent response (intermediate orientation). We allowed compass responses to adapt to the stationary polarizer and then presented the moving object (Fig. 2, B and C). In TB and CPU cells, each experiment included both preferred and antipreferred orientations. Most experiments also included testing at intermediate “neutral” orientations. Moving objects occurred in blocks of two to three stimuli, starting from 20 to 30 s after the presentation of the stationary E-vector had begun (Fig. 2C).
Responses to combined stimulation.
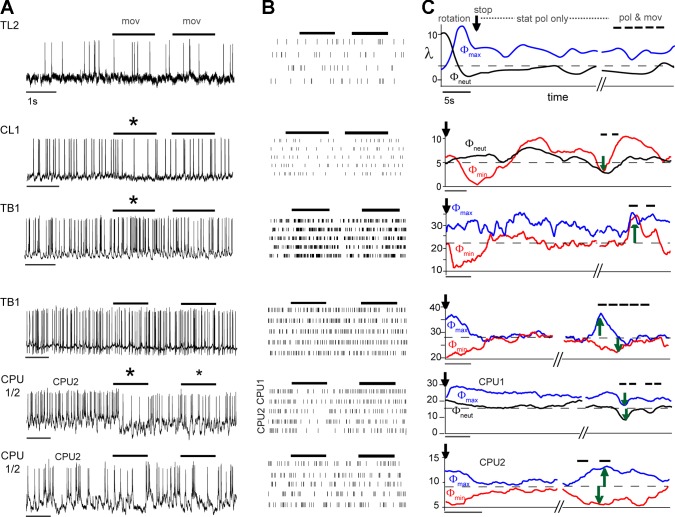
In TL2 cells (n = 4), moving-object stimuli had no effect on the cells’ strong tonic responses to their preferred E-vector nor did they trigger any response when being presented alone (Fig. 3, row 1). CL1 cells (n = 3), situated at the intermediate processing stage, showed inhibitory responses to the moving objects alone (Bockhorst and Homberg 2015b; Fig. 3, row 2). Following stop of the polarizer, CL1 cells showed adaptation of responses to polarized light followed by slow changes of activity around the average prestimulus activity (Fig. 3C, row 2). Nevertheless, the responses to moving objects were preserved during concurrent compass stimulation with polarized light, irrespective of the E-vector presented (Fig. 3C, row 2). Hence, whenever combined with an E-vector other than the antipreferred one, which in itself led to inhibition, the inhibitory response of the CL1 cell to motion interfered with the compass signaling function, as it changed the neuron’s firing rate in a direction that deviated from the currently expected compass response. Addition of motion-detection responses to the adapted compass responses also occurred in two out of five recorded TB1 neurons (Fig. 3C, row 3) and one out of two recorded CPU1 neurons (Fig. 3C, row 5). These cells responded to the moving object with excitation (TB1, Fig. 3C, row 3) and response inhibition (CPU1/2, Fig. 3C, row 5). Motion-detection responses during concurrent presentation of polarized light, therefore, corrupted the inhibitory compass responses in these TB cells and the excitatory compass responses in these CPU cells. Moreover, the effects of moving objects were rather transient in these cells, as previously reported for their novelty-dependent responses to presentation of the moving objects alone (Bockhorst and Homberg 2015b).
Fig. 3.
Spiking activity under uni- and bimodal stimulation. Recording traces (A) and rasterplots (B) show representative examples of responses to moving-object stimuli alone (mov/thick horizontal lines). Asterisks mark stimuli that evoked strong (large asterisk) or moderate (small asterisk) responses. C: the corresponding Gaussian-smoothed peristimulus time histograms illustrate how the same respective cell responded to a stationary polarizer (stat pol only) after precedent polarizer rotation stopped (vertical arrow) at an excitatory (Φmax, blue line), neutral (Φneut, black line), or inhibitory E-vector (Φmin, red line). Response to rotation is shown for the TL2 cell only (row 1); λ: firing rate (spikes/s). While some of the polarized-light responses decline to a rate close to the average prestimulus activity (thin horizontal dashed line), others stabilize at a residual plateau (excitatory responses of the TL2 cell and the excitatory responses of TB1 and CPU1 in rows 3 and 4). Occasionally, both cases seem to occur in the same cell (TB1, row 3). In contrast, the activity of the CL1 cell (row 2) following stop of polarizer rotation is marked by slow changes in firing rate both at the inhibitory and neutral E-vector. After stabilization of neural activity the moving object was presented while the presentation of stationary polarized light continued (pol and mov; thick black horizontal bars). With respect to moving object stimuli TL2 and CL1 cells behaved rather stereotypically, with no responsiveness to moving objects alone and combined stimulation in TL2 and a generalized inhibitory response to both scenarios involving the moving object in CL1. By contrast, two different response behaviors were observed in TB1 cells. TB1 cells responsive to the moving object alone (A, row 3, large asterisk) responded stereotypically, i.e., E-vector independently and in the same manner under combined stimulation (C, pol and mov). In TB1 cells unresponsive to the moving object alone (row 4), the combined stimulation triggered a regain of full compass responses, bringing back the unadapted levels of inhibitory or excitatory E-vector responses. The same dichotomy was observed in CPU cells. CPU1/2 neurons that were inhibited during object movement alone (row 5, A and B) maintained inhibitory responses during combined polarizer/moving object stimulation as illustrated for a CPU1 neuron in C. CPU1/2 neurons unresponsive to the moving object alone, however, showed disadaptation of the compass responses when stimulated with a moving object during concurrent presentation of the stationary polarizer as illustrated for a CPU2 neuron (row 6).
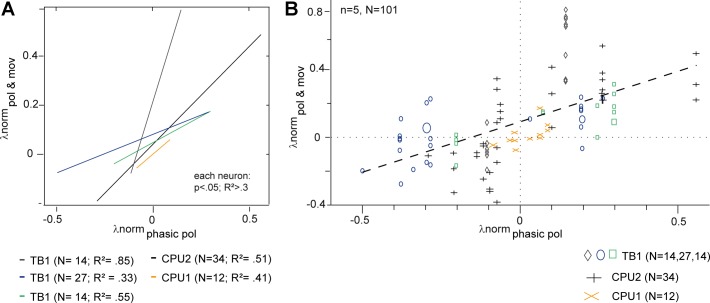
By contrast, six other cells (3 TB1 neurons, 1 CPU1 neuron, and 2 CPU2 cells) showed a substantially different response behavior. They were unresponsive to the moving object when presented alone (Fig. 3, A and B, rows 4 and 6). However, during stationary compass stimulation, moving objects had an effect that was correlated with the nonadapted, initial compass response (Fig. 3C, rows 4 and 6). That is, the effect of the same object-motion stimulus changed from inhibition to excitation, when the concurrently presented E-vector was changed from an antipreferred to a preferred one (Fig. 3C). In line with this, no effect of moving objects occurred during stimulation with a “neutral” E-vector orientation (Fig. 4). Hence, these cells regained their compass response when a rapidly moving object was presented in the visual periphery. Initial compass responses and disadapted ones were comparable in duration and much longer than the responses to moving objects alone that were observed in other cells (Fig. 3C). In some cases, the disadapted compass responses lasted even longer than the initial ones (Fig. 3C, row 6). To assess the correlation between response amplitudes, normalized amplitudes of initial compass responses were plotted against the responses induced by the combined stimuli (pol and mov). Correlation analyzes confirmed strong, highly significant linear associations in five out of the six neurons (Fig. 4A). The linear regression for response amplitudes pooled across all neurons had a positive slope of 0.6 (Fig. 4B), but individual neurons showed considerable variation in slope ranging from 0.3 to 2.5 (Fig. 4A). The sixth neuron, a CPU2 cell, showed a nonsignificant positive trend (not shown). Occasional presentations of optic flow in the form of many patches moving together did not trigger motion responses and failed to cause gain modulation in cells that showed gain modulation in response to salient single-patch motion (data not shown).
Fig. 4.
Correlation between the amplitudes of the nonadapted initial compass response and the response during combined E-vector/moving object stimulation in 5 gain-modulating neurons. A and B show normalized firing rates (λnorm; change from reference rate divided by reference rate; see Fig. 2C) during responses to combined stimulation (pol and mov) plotted against the unadapted compass responses measured right after the stop of polarizer rotation (phasic pol). A: linear regressions show significant (P < 0.05) positive correlation of normalized firing rates in 5 individual neurons. Colors code the different neurons; n = number of trials; R2 = coefficient of determination. B: same data set, but each data point represents 1 trial, measured in the neuron indicated by the plot marker legend below. Larger size symbols indicate 2 and in 1 case 3 identical data points. Dashed line: overall linear regression model; λnorm(pol and mov) = 0.6 λnorm(phasic pol) + 0.09; P ≪ 0.0001; R2 = 0.34.
Network model.
We have previously proposed a neural wiring scheme to explain how TB1 and CPU1/2 neurons can respond to preferred (Φmax) and antipreferred (Φmin) E-vector angles, while CL1 cells of the input stage solely respond at their Φmin (Bockhorst and Homberg 2015a). This scheme, however, was hitherto not validated. We now implemented the wiring pattern as a network of leaky integrate-and-firing neurons (Fig. 5) and expanded it to model the responses to moving targets. When run with either phasic or phasic-tonic polarized-light responses of CL1 cells as input, the model succeeded to reproduce the previously reported polarized-light responses of TB1 and CPU1/2 neurons. When responses to a moving-object where added at the CL1 level, the model produced either of the two response behaviors of TB and CPU cells reported in the current study, with the type of behavior depending on whether polarized-light responses of CL1 cells were phasic or phasic-tonic. Summation of compass- and object-motion responses concurred with phasic compass responses of the CL1 cell (Fig. 6C) and gain modulation concurred with phasic-tonic ones (Figs. 5 and 6, A and B).
Fig. 5.
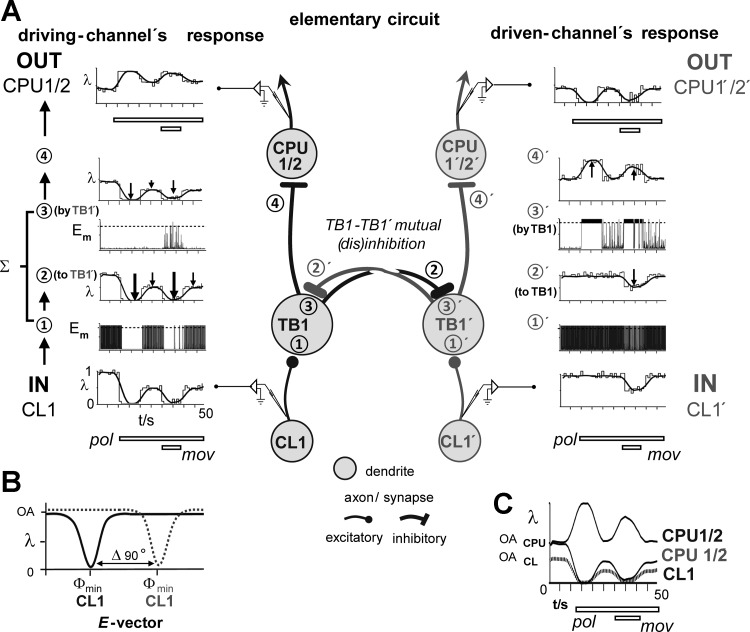
A network model explaining all types of response to separate and combined stimulation by compass signals and moving object stimuli. A: the elementary circuit in the center explains how TB1- and CPU1/2 neurons can respond to preferred (Φmax) and antipreferred E-vector angles (Φmin), while CL1 cells of the input stage solely respond at their Φmin (Bockhorst and Homberg 2015a). Two channels tuned to E-vector angles differing by 90° are interconnected through mutual inhibition between the 2 TB1 cells (TB1, TB1′) involved. A 2nd inhibition, situated at the TB1-CPU1/2 and TB1′-CPU1′/2′ connection, explains why preferred E-vectors of CPU1/2 cells and corresponding TB1 cells differ by 90° (Heinze and Homberg 2007). A, left (right): intracellular activity at crucial sites in the driving (driven) channel in terms of spike rate (λ, illustrated as Gaussian-smoothed and conventional peristimulus time histogram) or membrane potential (Em, dashed horizontal line: spike threshold). Data were obtained from a run of the simulation that used the input-activity (IN) shown for the CL1- and CL1′ cell at the bottom and produced the output (OUT) shown for the CPU1/2- and CPU1′/2′ cell at the top. As illustrated in B, the E-vector presented above the animal’s head evokes a maximal inhibitory response in the CL1 cell of the driving channel (left), while its counterpart in the driven channel (right; CL1′) is unresponsive to it, preserving its level of ongoing activity (OA). C: taken together, the circuit mediates the regain of declined E-vector responses under concurrent presentation of a moving object stimulus (mov) as well as contrast enhancement of the compass response to polarized light (pol) itself, in that CPU1/2 and CPU1′/2′ respond in an opponent manner and with a higher absolute change in firing rate because their level of ongoing activity is higher than in CL1 cells (Bockhorst and Homberg 2015a).
Fig. 6.
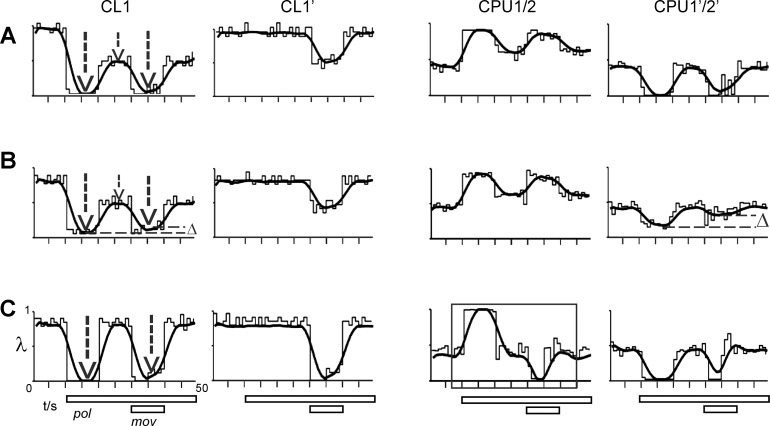
Variations in the responses of CL1 result in 3 different types of downstream-response to combined stimulation by compass and moving object stimuli. Conventions as in Fig. 5; plots of firing rate (λ) vs. time show the response of the CL1 cell in the driving (CL1) and driven (CL1′) channel and the corresponding output responses of the CPU1/2 and CPU1′/2′ cell. CPU1/2 responses are strongly determined by the temporal dynamics of the CL1 responses to polarized light. A: a strong phasic-tonic compass response in CL1 (1st and 2nd vertical arrow) results in an equally strong response to combined stimulation (3rd arrow) as well as in a regain of compass responses in CPU1/2 and CPU1′/2′ (reproduced from Fig. 5). B: a decrease in the tonic CL1 response (compare lengths of vertical arrows) leads to dampening (Δ, horizontal line) of the CL1 response to combined stimulation and of the regain of compass response in the driven CPU1′/2′. This in turn should correspond to a shallower slope of the regression lines in Fig. 4A. C: when the CL1 response to polarized light is purely phasic, inhibitory responses to the moving object occur in both CPU1/2 (box) and CPU1′/2′.
Figure 5A, middle column, illustrates the elementary wiring scheme that covers two interconnected CL-TB-CPU lines. Each of these lines can be regarded as a “polarotopic channel” that (anti-)prefers a particular E-vector angle. Importantly, the two interconnected channels are tuned to exactly opponent (Δ 90°) E-vector angles, meaning that, when the CL cell is maximally inhibited by the current E-vector, its partner in the interconnected channel, CL′, preserves its ongoing activity as the current E-vector is neutral to it (Fig. 5B). As a consequence, any compass response of the TB′- or CPU′ cell in the CL′-fed channel must be driven by activity in the CL-fed channel. The CL-fed, resp. CL′-fed channel in Fig. 5A is, therefore, dubbed “driving channel” and “driven channel,” respectively. To preserve the unidirectional compass responses at the CL-stage (no excitatory E-vectors, just inhibition at Φmin), the interconnection that transmits compass responses from the driving to the driven channel was positioned at the TB-TB′ level, affecting only the TB′- and downstream-connected CPU′ cell. As opponent responses to the same compass signal are required for the two channels, the cross-channel interaction is mutual inhibition. A second inhibition was implemented within each channel at the TB-CPU (TB′-CPU′) connection to take into account that preferred E-vectors of CPU1/2 cells and corresponding TB1 cells differ by 90° (Heinze and Homberg 2007).
In the following, the flow of activity in the network is described for the strong gain modulation type of response to combined stimulation by polarized light and the moving-object stimulus (see Fig. 6 and further below for mild gain modulation and summation behavior). Depicted in Fig. 5A, bottom left, the driving channel’s CL1 cell (IN CL1) responds to polarized light (pol) with phasic-tonic inhibition. As a consequence, its inhibitory response to the later added moving-object stimulus (mov) sums to the tonic part of the inhibition, resulting in maximum inhibition upon combined stimulation. This response is preserved at the excitatory CL1-TB1 synapse (① in Fig. 5A, left and middle column). At the inhibitory TB1-TB1′ connection, an inverted (disinhibiting) copy of this response is fed into the driven channel’s TB1′ cell, bringing it to net excitation upon polarized light-stimulation and combined stimulation (②,③′).
In the driven channel, the moving-object response of the CL1′ cell rides on a preserved high-level ongoing activity, because no response to polarized light occurs. As a result, the connected TB1′cell remains strongly activated and thus exerts a strong inhibition onto the TB1 cell at the TB1′-TB1 synapse (②′,③), which is barely reduced during presentation of the moving object. Summation of postsynaptic activity at ① and ③ (①′ and ③′) produces the spiking output at ④ (④′), which is sign inverted again at the inhibitory synapse onto CPU1/2 and CPU1′/2′. This finally leads to excitation of the driving channel’s CPU1/2 cell by disinhibition and to pronounced inhibition of the driven channel’s CPU1′/2′ cell.
Variability of the gain-modulating effect (at the same E-vector; Fig. 4) likely traces back to the long-term dynamics of ongoing activity in the CL1 cells (Bockhorst and Homberg 2015a, 2015b). As illustrated in Fig. 6, A and B, the strength of gain modulation in the driven channel decreases when the residual plateau of CL1 firing in the driving channel is increased. Plots in Fig. 6A replicate the situation explained in Fig. 5, with a strong inhibitory CL1 response to combined stimulation resulting in strong regain of the compass response in the CPU1/2 and CPU1′/2′ cell. A decrease of the tonic CL1 response to polarized light (Fig. 6B), also leading to a decreased inhibition upon combined stimulation (Fig. 6B, dashed lines), results in less pronounced regain of the compass response in CPU1′/2′ (Fig. 6B, dashed horizontal lines). When the CL1 response to polarized light is purely phasic and responses to combined stimulation are equally strong in CL1 and CL1′ (Fig. 6C), an override of compass signaling by a stereotypic response to object motion can occur (CPU1/2, box). This is caused by an equally strong disinhibition at both the TB1-TB1′ and the TB1′-TB1 synapse that produces high rates of firing at the postsynaptic sites ③ and ③′ in Fig. 5A. Due to the sign inversion at the TB1-CPU1/2 and TB1′-CPU1′/2′ synapse, this results in inhibitions in both the CPU1/2 and the CPU1′/2′ cell upon presentation of the moving object during ongoing presentation of polarized light. The comparison of Fig. 6, A–C, illustrates that CPU1/2 responses are strongly determined by the temporal dynamics of the CL1 responses to polarized light, more precisely by whether these are strong and phasic-tonic or less strong and phasic.
DISCUSSION
Visual pathways underlying adaptive orientation behavior in insects.
Animal survival critically depends on spatial orientation for goal-directed locomotion (Mouritsen 2001) as well as on the ability to respond to unexpected events. In various insect species, a role in goal-directed locomotion has been assigned to a particular brain area, the central complex (Bech et al. 2014; Bockhorst and Homberg 2015a; el Jundi et al. 2015; Heinze and Homberg 2007; Heinze and Reppert 2011; Pfeiffer and Homberg 2014; Sakura et al. 2008; Seelig and Jayaraman 2015; Varga and Ritzmann 2016). Our previous work revealed that sky-compass cells in this brain region respond to virtual objects moving through the visual scene (Bockhorst and Homberg 2015b). Responses to moving objects reflected the novelty character of the stimulus rather than specific object features and resembled those to simulated object approach (looming) in duration and the occurrence of stimulus-specific adaptation (Bockhorst and Homberg 2015b; Rosner and Homberg 2013). Both looming and translating objects drive a fast descending pathway that triggers escape jumps and glides in the locust (Gabbiani et al. 2001; Rind and Bramwell 1996; Rowell et al. 1977; Santer et al. 2006; Simmons 1980), but the resultant behavior was studied largely for looming objects (Chan and Gabbiani 2013; Ribak et al. 2012; Robertson and Johnson 1993). The pathway’s motion detector neuron, termed lobula giant motion detector (LGMD), receives input via dendrites that span the width of the third neuropil in the optic lobe. This dendritic architecture allows the LGMD to track small-field motion throughout a wide section of the visual field, whereas the cell ignores stereotypic flow of the optic background, resulting from ego motion in nature and mimicked by coherent translations of stripes or patches (Rowell et al. 1977). A specialized synapse rapidly and faithfully transmits every LGMD spike onto the descending contralateral motion detector (DCMD), which in turn activates metathoracic motor neurons (reviewed by Fotowat and Gabbiani 2011).
Various studies, including an elegant combination of electrophysiology and motion tracking in unrestrained locusts (Fotowat et al. 2011), have characterized the LGMD-DCMD-mediated signaling of fast object approach (looming), a threatening stimulus that triggers escape jumps in sitting/walking locusts and escape glides in flying subjects. Recent work has addressed the detection of small-field motion along more natural, complex trajectories that include transitions from translation to looming (Dick and Gray 2014; McMillan and Gray 2012; Silva et al. 2015; Yakubowski et al. 2016). While imminent collision may be required to maximally drive the DCMD and finally trigger escape, the precedent tracking of more distant translational motion can already raise spike rate from near-zero spontaneous activity to levels well above 100 spikes/s (Fotowat et al. 2011; Silva et al. 2015; Yakubowski et al. 2016). This can suffice to time the cocontraction/flexion of leg muscles that serves a critical role in the preparation of evasive maneuvers, as even reflexive escape jumps take several hundred milliseconds to occur (Santer et al. 2008). Moreover, locomotor states, in particular flight, further increase the level of ongoing activity in the DCMD neuron (Rind et al. 2008).
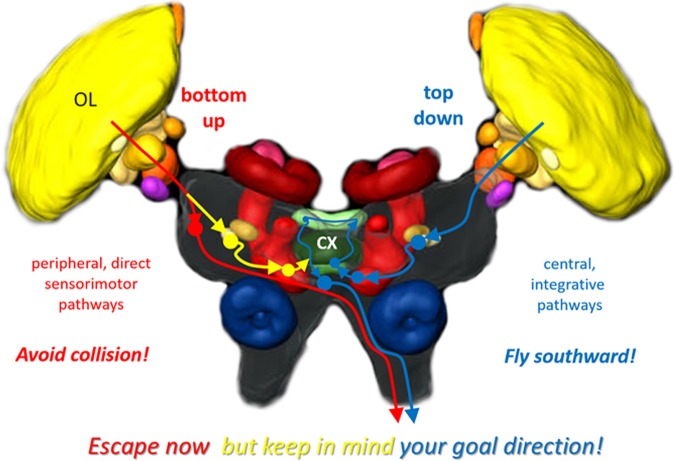
Compass cells in the locust central complex also respond to translations and looming of small-field objects (Bockhorst and Homberg 2015b; Rosner and Homberg 2013). In fact, response features for translational object motion are very similar between the LGMD-DCMD system and the central complex, in that responses are confined to small-field objects and are novelty dependent and modulated by how salient the moving object stands out against the optic background (Bockhorst and Homberg 2015b; O’Shea and Rowell 1975; Silva et al. 2015; Yakubowski et al. 2016). This strongly suggests that both systems are fed by very similar or the same early stage motion detectors and contribute to the behavioral consequences of the same salient or threatening visual events. The compass cells we encountered were equally responsive to translation and looming (Bockhorst and Homberg 2015b; Rosner and Homberg 2013); the responses actually look the same, whereas DCMD cells appear to be more sensitive to looming (McMillan and Gray 2012; Silva et al. 2015). This difference in neuronal responses may reflect the different roles of both systems in controlling goal-directed locomotion. The DCMD activity-related jumps and glides in response to looming objects ensure safe escape under acute danger of collision. In addition, timely detection of a moving object allows early cocontraction of leg muscles as well as the disadaptation of the compass system to prepare for more planned maneuvers whenever possible. Figure 7 illustrates a working hypothesis on how these two levels of adaptive locomotor behavior are implemented in the locust brain.
Fig. 7.
Working hypothesis on visual pathways for adaptive goal-directed behavior in the locust brain. Pathway in red represents fast routes of sensory-motor transformation that circumvent the central complex (CX) to mediate reflexive escape, such as the LGMD-DCMD system that serves to avoid collision. The blue pathway represents the polarization-vision pathway for sky compass orientation, which involves the central-complex network. This pathway serves to integrate long-term goals in planned locomotion in a top-down fashion, e.g., to ensure southward directed migration in spring. Both pathways eventually descend to metathoracic motor centers. A link between both pathways, shown in yellow, serves to inform the top-down planning system on acute events such as imminent collision that will trigger an escape in the near future. This interconnection between both pathways allows the animal to “escape now, but keep in mind your goal direction” for the subsequent resumption of migration in a particular compass direction. Arrowheads and filled circles symbolize pre- and postsynaptic sites, respectively. OL, optic lobe. Brain scheme modified from el Jundi et al. (2010).
Responses to combined object motion and compass stimuli.
In the experiments reported here, we asked the following: how does a neural circuit responsible for compass-guided long-range migration integrate acutely relevant sensory stimuli that draw the animal’s attention and require direct motor action? The stimulus regime applied here resembles a sensory situation in which the locust aligns to a particular compass course, keeps this course for a while, and is then confronted with a moving object. The latter could correspond to a predator or a conspecific when flying in a swarm, both of which would most likely cause arousal and trigger evasive maneuvers (whereas experiments in the present study were performed in immobilized animals with legs and wings removed). The salient event stimulus (moving object) generally had an effect on the spiking of compass cells while the animal was receiving the compass stimulus, polarized light, from above. What differed between the recorded neurons is how the two types of information, heading direction vs. a novel event in the visual environment, were integrated with each other. Some neurons responded to the object irrespective of concurrent E-vector orientation, while in others object presentation restored a previously adapted compass response. The behavioral consequences of both response types will have to be determined. If a moving object elicits similar responses in all compass neurons of a given type such as CPU cells that code for all possible E-vector orientations across the slices of the protocerebral bridge (Fig. 1C), the highly correlated object responses in these neurons might basically correspond to added “noise” that could easily be removed by downstream circuits for sensory-motor transformation, e.g., by mutual inhibition between channels tuned to opponent heading directions.
In contrast, the disadaptation of compass responses seen in the second response type might be highly relevant to preserve goal-directed locomotion. It might guide evasive maneuvers or help to get back on course afterwards. Bringing up a representation of the original compass bearing, possibly right before an escape maneuver, might provide a basis for a memory template for subsequent reorientation to the desired compass course. If the information is read and stored by another neural subsystem, the acute heading direction after escape could be compared with the memory template of the original, aspired compass course to control corrective yaw and get back on course. This scenario would be in line with previous implications on a role of the central complex for visuospatial working memory (Neuser et al. 2008).
Variability of response-gain modulation.
The amplitudes of initial compass responses and the corresponding disadapted ones were linearly correlated, but the slopes for different recordings substantially differed (Fig. 4A). The behavior of the neural network model suggests that smaller slopes can result from an increased level of ongoing activity in CL1 cells, which could lead to an unbalanced dampening of responses to polarized light and combined stimulation (Fig. 6B). In principle, a very shallow or steep slope of the fit line should not corrupt the information content of the disadapted population response as long as the change in amplitude is comparable across output channels of the compass network. Then, the most strongly driven channel should still be the same as for the initial compass response even if the absolute amplitude of the compass response has changed. Thus valid compass signaling should be possible also for those cells whose linear slopes differ from 1, if sensory-motor transformation includes evaluation of the population response by comparison of the activity across channels.
Network modeling.
We presume that the two types of response represent different subranges within a continuum between strong gain modulation and mere summation of compass- and object-motion responses, i.e., these response types are operational states of the same network rather than two functionally distinct cellular populations. The CL-TB-CPU wiring scheme (Figs. 5 and 6), originally developed to explain features of pure compass responses (Bockhorst and Homberg 2015a), is suited to explain both types of responses to combined stimulation described here. In particular, gain modulation of compass responses upon object motion occurred when the simulated compass responses of CL cells included a tonic component. Such phasic-tonic responses to prolonged compass stimulation were in fact observed concurrent with gain modulation. This could indicate that the disadaptation effect requires the compass network to be in a state of increased responsiveness to prolonged reception of compass stimuli, possibly reflecting an animal’s urge to navigate.
In methodological respects, the network model is simple in design, including only well-known cell types, connected by simple synapses into a processing hierarchy supported by abundant anatomical and physiological data (Heinze and Homberg 2007; Homberg et al. 2009, 2011). Key processing steps are based on well-established concepts of vertical and lateral disinhibition. The model is capable of explaining aspects of the responses to polarized light only (only inhibitory responses in CL cells but inhibitory and excitatory responses in TB and CPU cells), as well as aspects of responses to moving-object stimuli (inhibitory in CL and CPU cells but excitatory in TB cells). Finally, it is suited to explain the two types of response to combined stimulation, including the continuum of response strength for the gain modulation effect, based on differences in the state-like ongoing activity of CL cells.
Parallels to vertebrates.
The neuronal processing of compass signals and visual objects in the central brain of the locust shares several key features with higher sensory processing in vertebrate brains. We have previously shown that compass responses observed in locust central-complex neurons share features with responses of rat head-direction cells, e.g., with respect to tuning profiles and a dependency of responses on the direction of head rotation which serves to anticipate upcoming heading directions during simulated steering (Bockhorst and Homberg 2015a). Our approach to explain the responses of the present study by neural modeling resulted in a model based on mutual inhibition between processing channels with opponent compass tuning, which parallels the principle of ring-attractor models for networks of head direction cells in rats (Clark and Taube 2012). The model also relies on modulation of evoked activity (here at the input layer, i.e., CL1 cells) by the dynamics of ongoing activity or “brain states,” a phenomenon of fundamental importance in vertebrate cortex as well (Bockhorst and Homberg 2015a, 2015b; Kaiser and Steiner-Kaiser 1983; Lin et al. 2015; Perez-Orive et al. 2002; Sadaghiani et al. 2010). Finally and as pointed out earlier (Bockhorst and Homberg 2015b), the rapid adaptation of responses to repetitive stimulation, termed stimulus specific adaptation, is a phenomenon shared with higher sensory processing in vertebrates that allows separating novel events in the sensory landscape from a dynamic background scenery (Bockhorst and Homberg 2015b; Gutfreund 2012). In light of such profound parallels, it would be interesting to see how vertebrate systems for navigation such as head-direction cells in rats respond to startle of the locomoting animal, e.g., by loud sounds or bright flashes.
In a study that comprises histological, physiological, ethological, and genetic data, the central complex was considered a homolog of the basal ganglia, responsible for action selection and skill learning (Strausfeld and Hirth 2013). Our current results as well as a variety of complementary work show how effectively the central complex integrates information from very different modalities, e.g., compass signals and visual threat detection, to control adaptive behavior that relates the subject to its environment in ways crucial to species survival. This process draws on visual pattern memory (Liu et al. 2006; Pan et al. 2009), visual-spatial working memory (Neuser et al. 2008), head-direction coding (Pfeiffer and Homberg 2014; Seelig and Jayaraman 2015), place learning (Ofstad et al. 2011), sleep homeostasis (Ueno et al. 2012), and valency coding (Beshel and Zhong 2013). In summary, the data currently available identify the central complex as a processing site in the insect brain that integrates goals, internal states, and exteroceptive input to control adaptive behavior at different timescales, from escape from acute threat to ongoing navigation in long-range migrations. The central complex of the insect brain may thus be considered a functional analog of limbic/cortical networks (e.g., the prefrontal-cingulate network) that link behavior to goals by means of executive functions.
GRANTS
This work was supported by Deutsche Forschungsgemeinschaft Grants HO-950/16-3 and HO-950/23-1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.B. and U.H. conceived and designed research; T.B. performed experiments; T.B. analyzed data; T.B. interpreted results of experiments; T.B. and U.H. prepared figures; T.B. drafted manuscript; T.B. and U.H. edited and revised manuscript; T.B. and U.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. Keram Pfeiffer for MATLAB scripts used to calculate and plot the polarization pattern in Fig. 1A. We thank Jonas Hollensteiner for helpful comments on an earlier version of the manuscript.
REFERENCES
- Bech M, Homberg U, Pfeiffer K. Receptive fields of locust brain neurons are matched to polarization patterns of the sky. Curr Biol 24: 2124–2129, 2014. doi: 10.1016/j.cub.2014.07.045. [DOI] [PubMed] [Google Scholar]
- Beshel J, Zhong Y. Graded encoding of food odor value in the Drosophila brain. J Neurosci 33: 15693–15704, 2013. doi: 10.1523/JNEUROSCI.2605-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockhorst T, Homberg U. Amplitude and dynamics of polarization-plane signaling in the central complex of the locust brain. J Neurophysiol 113: 3291–3311, 2015a. doi: 10.1152/jn.00742.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockhorst T, Homberg U. Compass cells in the brain of an insect are sensitive to novel events in the visual world. PLoS One 10: e0144501, 2015b. doi: 10.1371/journal.pone.0144501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RW, Gabbiani F. Collision-avoidance behaviors of minimally restrained flying locusts to looming stimuli. J Exp Biol 216: 641–655, 2013. doi: 10.1242/jeb.077453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BJ, Taube JS. Vestibular and attractor network basis of the head direction cell signal in subcortical circuits. Front Neural Circuits 6: 7, 2012. doi: 10.3389/fncir.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements AN, May TE. Studies on locust neuromuscular physiology in relation to glutamic acid. J Exp Biol 60: 673–705, 1974. [DOI] [PubMed] [Google Scholar]
- Dick PC, Gray JR. Spatiotemporal stimulus properties modulate responses to trajectory changes in a locust looming-sensitive pathway. J Neurophysiol 111: 1736–1745, 2014. doi: 10.1152/jn.00499.2013. [DOI] [PubMed] [Google Scholar]
- el Jundi B, Heinze S, Lenschow C, Kurylas A, Rohlfing T, Homberg U. The locust standard brain: a 3D standard of the central complex as a platform for neural network analysis. Front Syst Neurosci 3: 21, 2010. doi: 10.3389/neuro.06.021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Jundi B, Warrant EJ, Byrne MJ, Khaldy L, Baird E, Smolka J, Dacke M. Neural coding underlying the cue preference for celestial orientation. Proc Natl Acad Sci USA 112: 11395–11400, 2015. doi: 10.1073/pnas.1501272112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotowat H, Gabbiani F. Collision detection as a model for sensory-motor integration. Annu Rev Neurosci 34: 1–19, 2011. doi: 10.1146/annurev-neuro-061010-113632. [DOI] [PubMed] [Google Scholar]
- Fotowat H, Harrison RR, Gabbiani F. Multiplexing of motor information in the discharge of a collision detecting neuron during escape behaviors. Neuron 69: 147–158, 2011. doi: 10.1016/j.neuron.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani F, Cox SJ. Mathematics for Neuroscientists. London: Elsevier/Academic, 2010. [Google Scholar]
- Gabbiani F, Mo C, Laurent G. Invariance of angular threshold computation in a wide-field looming-sensitive neuron. J Neurosci 21: 314–329, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutfreund Y. Stimulus-specific adaptation, habituation and change detection in the gaze control system. Biol Cybern 106: 657–668, 2012. doi: 10.1007/s00422-012-0497-3. [DOI] [PubMed] [Google Scholar]
- Heinze S, Gotthardt S, Homberg U. Transformation of polarized light information in the central complex of the locust. J Neurosci 29: 11783–11793, 2009. doi: 10.1523/JNEUROSCI.1870-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze S, Homberg U. Maplike representation of celestial E-vector orientations in the brain of an insect. Science 315: 995–997, 2007. doi: 10.1126/science.1135531. [DOI] [PubMed] [Google Scholar]
- Heinze S, Reppert SM. Sun compass integration of skylight cues in migratory monarch butterflies. Neuron 69: 345–358, 2011. doi: 10.1016/j.neuron.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Homberg U, Heinze S, Pfeiffer K, Kinoshita M, el Jundi B. Central neural coding of sky polarization in insects. Philos Trans R Soc Lond B Biol Sci 366: 680–687, 2011. doi: 10.1098/rstb.2010.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W, Steiner-Kaiser J. Neuronal correlates of sleep, wakefulness and arousal in a diurnal insect. Nature 301: 707–709, 1983. doi: 10.1038/301707a0. [DOI] [PubMed] [Google Scholar]
- Kreuz T, Chicharro D, Greschner M, Andrzejak RG. Time-resolved and time-scale adaptive measures of spike train synchrony. J Neurosci Methods 195: 92–106, 2011. doi: 10.1016/j.jneumeth.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Labhart T, Meyer EP. Detectors for polarized skylight in insects: a survey of ommatidial specializations in the dorsal rim area of the compound eye. Microsc Res Tech 47: 368–379, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- Lin IC, Okun M, Carandini M, Harris KD. The nature of shared cortical variability. Neuron 87: 644–656, 2015. doi: 10.1016/j.neuron.2015.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Seiler H, Wen A, Zars T, Ito K, Wolf R, Heisenberg M, Liu L. Distinct memory traces for two visual features in the Drosophila brain. Nature 439: 551–556, 2006. doi: 10.1038/nature04381. [DOI] [PubMed] [Google Scholar]
- McMillan GA, Gray JR. A looming-sensitive pathway responds to changes in the trajectory of object motion. J Neurophysiol 108: 1052–1068, 2012. doi: 10.1152/jn.00847.2011. [DOI] [PubMed] [Google Scholar]
- Mota T, Yamagata N, Giurfa M, Gronenberg W, Sandoz JC. Neural organization and visual processing in the anterior optic tubercle of the honeybee brain. J Neurosci 31: 11443–11456, 2011. doi: 10.1523/JNEUROSCI.0995-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen H. Navigation in birds and other animals. Image Vis Comput 19: 713–731, 2001. doi: 10.1016/S0262-8856(00)00110-4. [DOI] [Google Scholar]
- Neuser K, Triphan T, Mronz M, Poeck B, Strauss R. Analysis of a spatial orientation memory in Drosophila. Nature 453: 1244–1247, 2008. doi: 10.1038/nature07003. [DOI] [PubMed] [Google Scholar]
- O’Shea M, Rowell CHF. Protection from habituation by lateral inhibition. Nature 254: 53–55, 1975. doi: 10.1038/254053a0. [DOI] [PubMed] [Google Scholar]
- Ofstad TA, Zuker CS, Reiser MB. Visual place learning in Drosophila melanogaster. Nature 474: 204–207, 2011. doi: 10.1038/nature10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Zhou Y, Guo C, Gong H, Gong Z, Liu L. Differential roles of the fan-shaped body and the ellipsoid body in Drosophila visual pattern memory. Learn Mem 16: 289–295, 2009. doi: 10.1101/lm.1331809. [DOI] [PubMed] [Google Scholar]
- Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science 297: 359–365, 2002. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- Pfeiffer K, Homberg U. Organization and functional roles of the central complex in the insect brain. Annu Rev Entomol 59: 165–184, 2014. doi: 10.1146/annurev-ento-011613-162031. [DOI] [PubMed] [Google Scholar]
- Pfeiffer K, Kinoshita M. Segregation of visual inputs from different regions of the compound eye in two parallel pathways through the anterior optic tubercle of the bumblebee (Bombus ignitus). J Comp Neurol 520: 212–229, 2012. doi: 10.1002/cne.22776. [DOI] [PubMed] [Google Scholar]
- Ribak G, Rand D, Weihs D, Ayali A. Role of wing pronation in evasive steering of locusts. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 198: 541–555, 2012. doi: 10.1007/s00359-012-0728-z. [DOI] [PubMed] [Google Scholar]
- Rind FC, Bramwell DI. Neural network based on the input organization of an identified neuron signaling impending collision. J Neurophysiol 75: 967–985, 1996. [DOI] [PubMed] [Google Scholar]
- Rind FC, Santer RD, Wright GA. Arousal facilitates collision avoidance mediated by a looming sensitive visual neuron in a flying locust. J Neurophysiol 100: 670–680, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson RM, Johnson AG. Collision avoidance of flying locusts: steering torques and behavior. J Exp Biol 183: 35–60, 1993. [Google Scholar]
- Rosner R, Homberg U. Widespread sensitivity to looming stimuli and small moving objects in the central complex of an insect brain. J Neurosci 33: 8122–8133, 2013. doi: 10.1523/JNEUROSCI.5390-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossel S, Wehner R. The bee’s E-vector compass. In: Neurobiology and Behavior of Honeybees, edited by Menzel R, Mercer A. Berlin, Germany: Springer-Verlag, 1987, p. 76–93. doi: 10.1007/978-3-642-71496-2_7. [DOI] [Google Scholar]
- Rowell CHF, O’Shea M, Williams JLD. The neuronal basis of a sensory analyser, the acridid movement detector system. IV. The preference for small field stimuli. J Exp Biol 68: 157–185, 1977. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, Hesselmann G, Friston KJ, Kleinschmidt A. The relation of ongoing brain activity, evoked neural responses, and cognition. Front Syst Neurosci 4: 20, 2010. doi: 10.3389/fnsys.2010.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakura M, Lambrinos D, Labhart T. Polarized skylight navigation in insects: model and electrophysiology of e-vector coding by neurons in the central complex. J Neurophysiol 99: 667–682, 2008. doi: 10.1152/jn.00784.2007. [DOI] [PubMed] [Google Scholar]
- Santer RD, Rind FC, Stafford R, Simmons PJ. Role of an identified looming-sensitive neuron in triggering a flying locust’s escape. J Neurophysiol 95: 3391–3400, 2006. doi: 10.1152/jn.00024.2006. [DOI] [PubMed] [Google Scholar]
- Santer RD, Yamawaki Y, Rind FC, Simmons PJ. Preparing for escape: an examination of the role of the DCMD neuron in locust escape jumps. J Comp Physiol A 194: 69–77, 2008. [DOI] [PubMed] [Google Scholar]
- Seelig JD, Jayaraman V. Neural dynamics for landmark orientation and angular path integration. Nature 521: 186–191, 2015. doi: 10.1038/nature14446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, McMillan GA, Santos CP, Gray JR. Background complexity affects response of a looming-sensitive neuron to object motion. J Neurophysiol 113: 218–231, 2015. doi: 10.1152/jn.00478.2014. [DOI] [PubMed] [Google Scholar]
- Simmons P. Connexions between a movement-detecting visual interneurone and flight motoneurones of a locust. J Exp Biol 86: 87–97, 1980. [Google Scholar]
- Strausfeld NJ, Hirth F. Deep homology of arthropod central complex and vertebrate basal ganglia. Science 340: 157–161, 2013. doi: 10.1126/science.1231828. [DOI] [PubMed] [Google Scholar]
- Ueno T, Tomita J, Tanimoto H, Endo K, Ito K, Kume S, Kume K. Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nat Neurosci 15: 1516–1523, 2012. doi: 10.1038/nn.3238. [DOI] [PubMed] [Google Scholar]
- Varga AG, Ritzmann RE. Cellular basis of head direction and contextual cues in the insect brain. Curr Biol 26: 1816–1828, 2016. doi: 10.1016/j.cub.2016.05.037. [DOI] [PubMed] [Google Scholar]
- Wehner R. Polarized-light navigation by insects. Sci Am 235: 106–115, 1976. doi: 10.1038/scientificamerican0776-106. [DOI] [PubMed] [Google Scholar]
- Wehner R, Labhart T. Polarization vision. In: Invertebrate Vision, edited by Warrant E, Nilsson DE. Cambridge, UK: Cambridge University Press, 2006, p. 291–348. [Google Scholar]
- Yakubowski JM, McMillan GA, Gray JR. Background visual motion affects responses of an insect motion-sensitive neuron to objects deviating from a collision course. Physiol Rep 4: e12801, 2016. doi: 10.14814/phy2.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]