Abstract
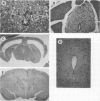
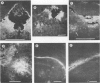
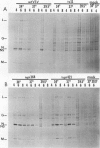
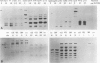
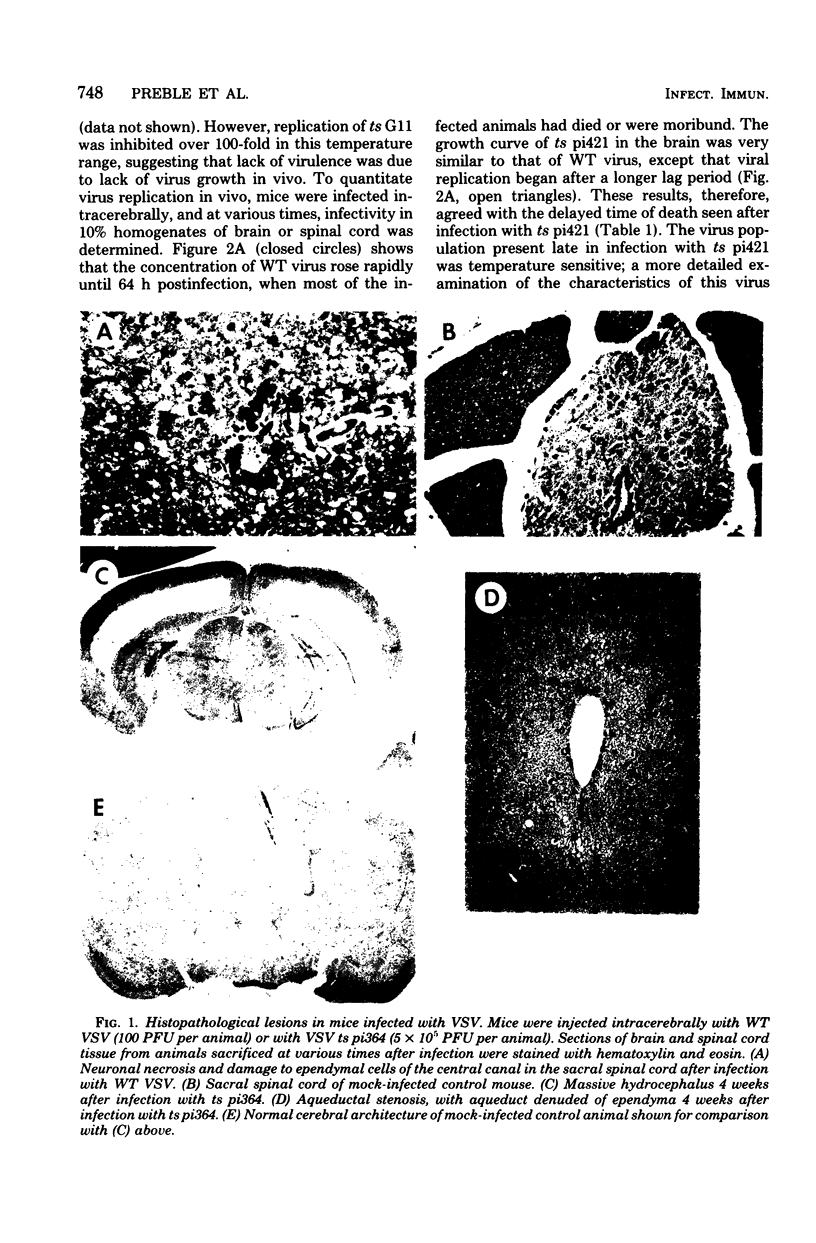
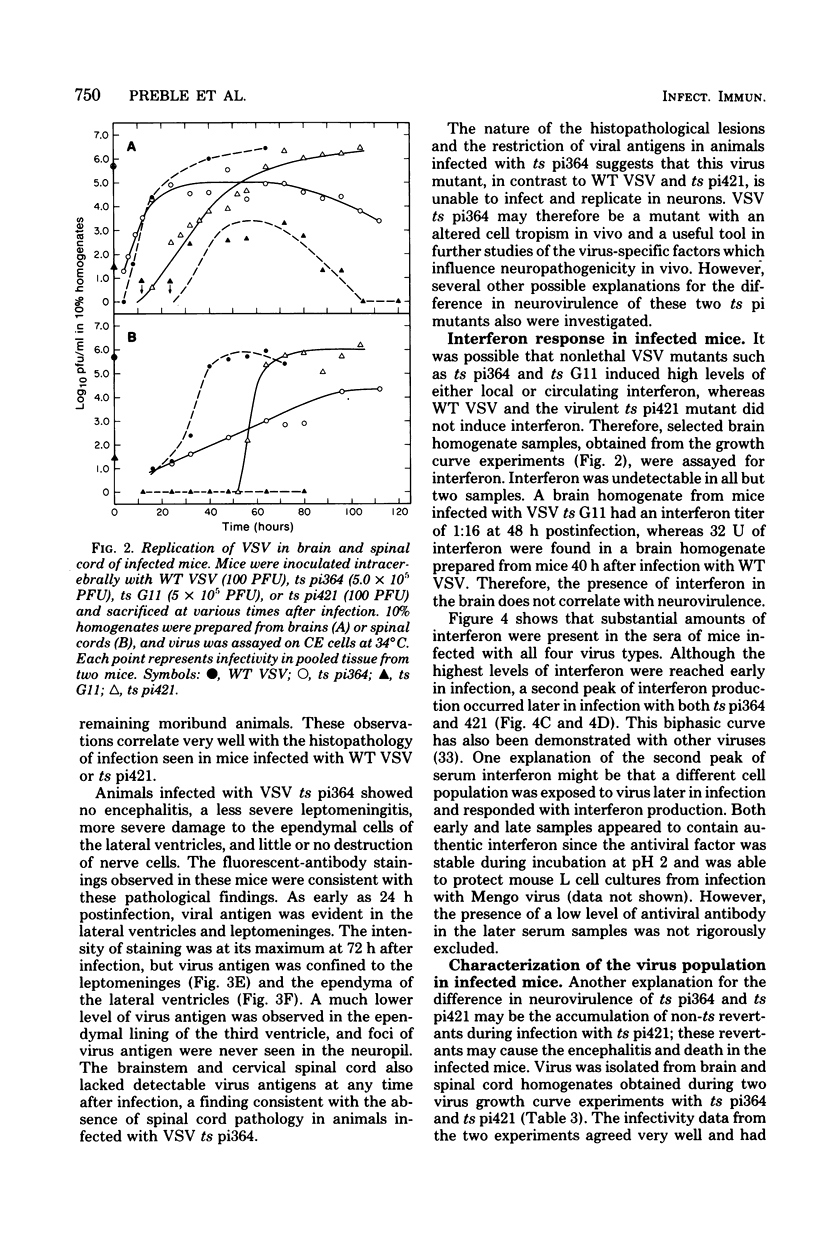
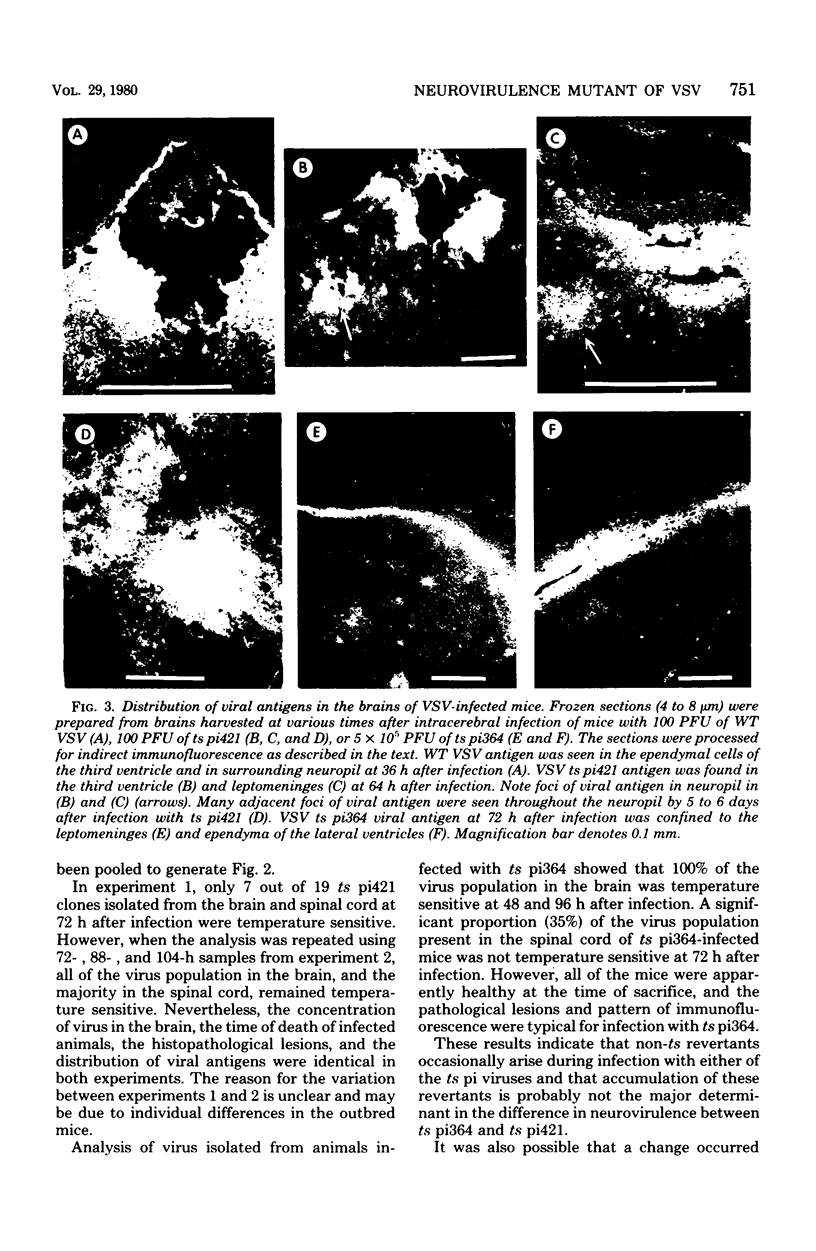
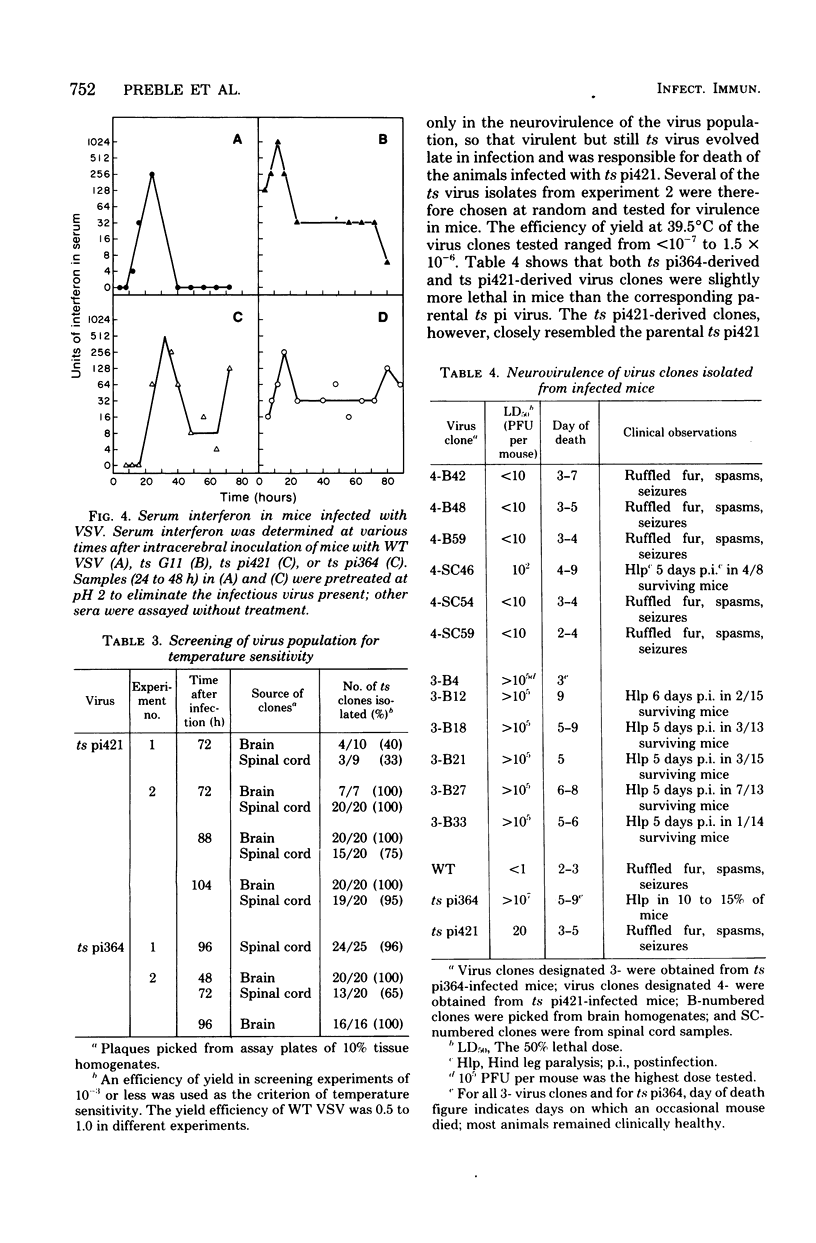
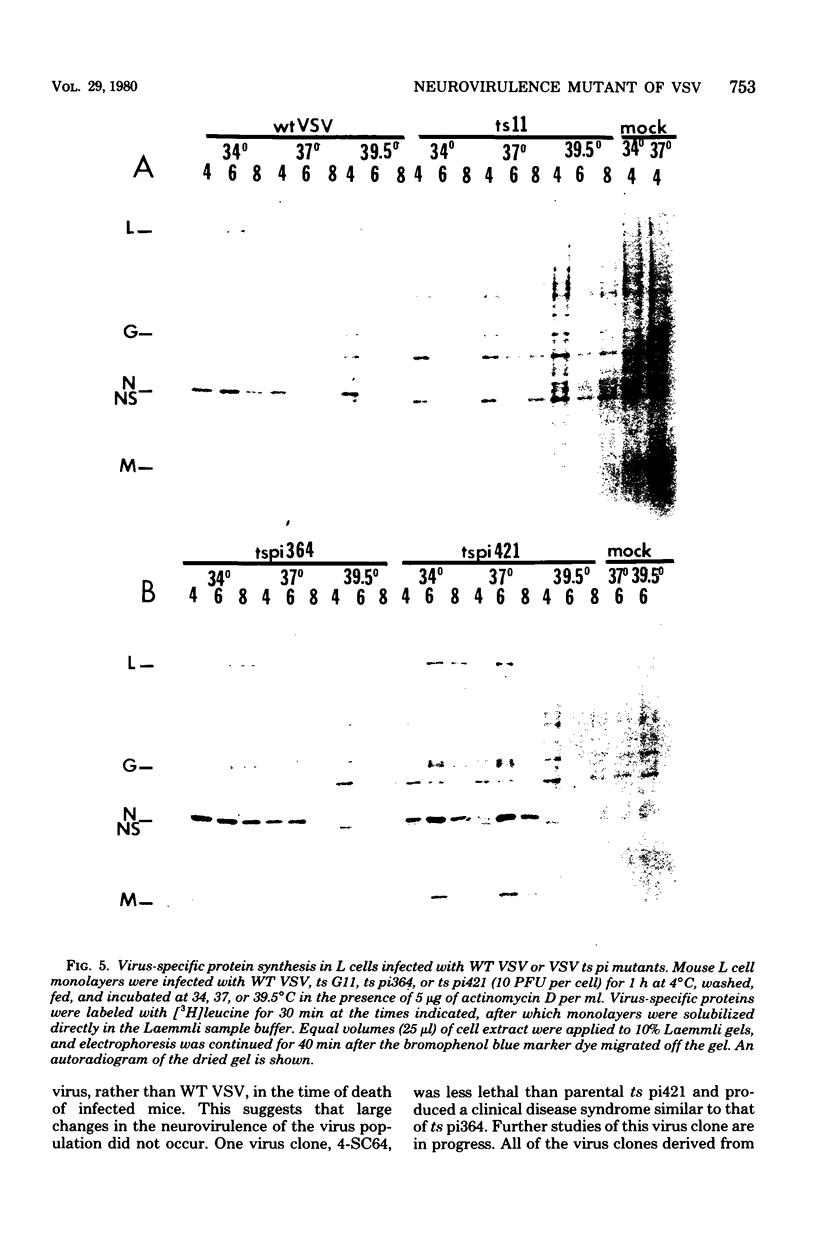
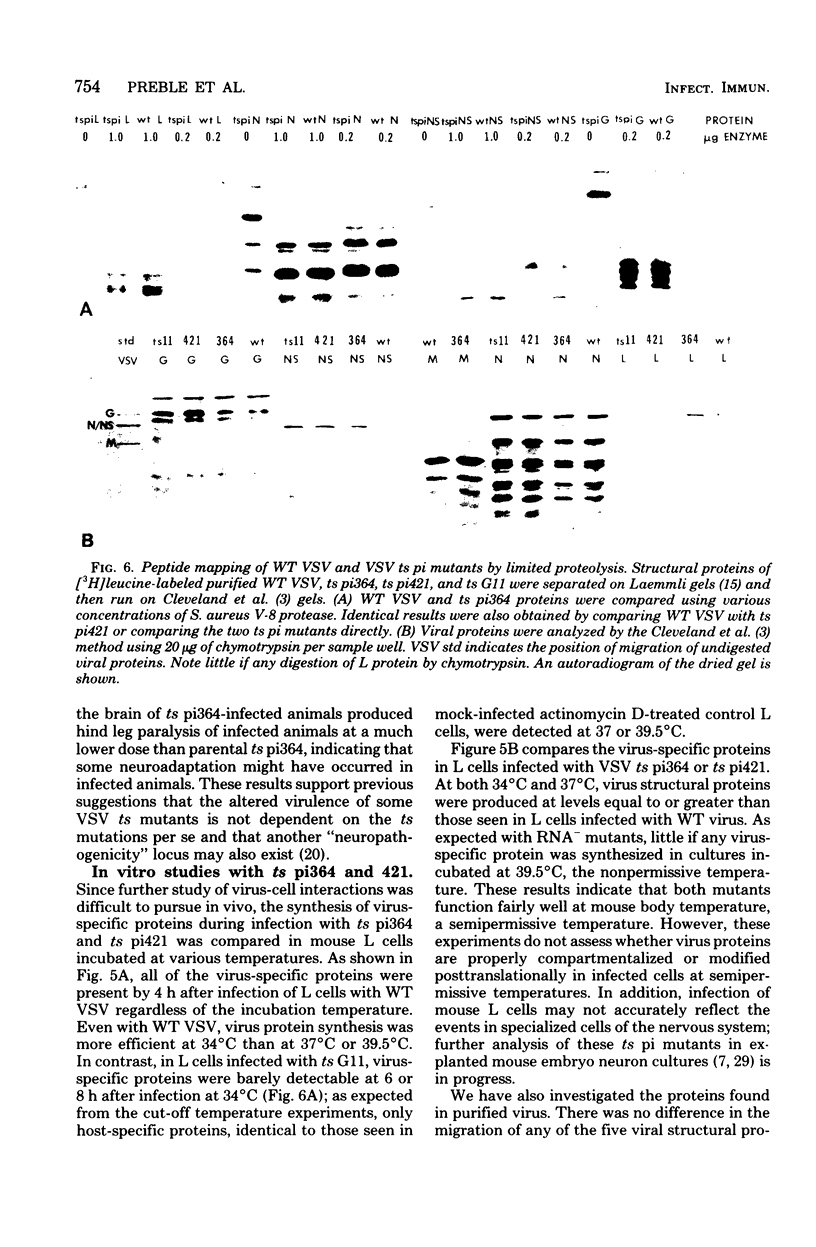
Intracerebral infection of weanling Swiss mice with a temperature-sensitive (ts) mutant of vesicular stomatitis virus (VSV), ts pi364, resulted in a unique neuropathological syndrome not previously described with other VSV mutants. Mice infected with wild-type VSV died from an acute encephalitis characterized by neuronal necrosis and efficient virus replication in both brain and spinal cord. In contrast, with VSV ts pi364, the most prominent histopathological feature was destruction of the ependyma of the lateral ventricles. Virus antigen was also limited to the leptomeninges and the lateral ventricles. Infected mice survived and developed hydrocephalus. Replication of ts pi364 in the brain was 10- to 100- fold less than that of wild-type VSV, and appearance of virus in the spinal cord was delayed. VSV ts pi364 was isolated from mouse cells persistently infected with VSV. Another VSV ts pi mutant, isolated from the same persistent infection, behaved in vivo like wild-type VSV, even though both mutants were very similar in plaque size, reversion frequency, cut-off temperature, and synthesis of virus-specific proteins at semipermissive temperature. These results strongly suggest that VSV ts pi364 has a second, non-ts mutation which results in a restricted target cell range in vivo; wild-type VSV can infect both neurons and ependymal cells, whereas ts pi364 does not replicate in neurons.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breschkin A. M., Haspel M. V., Rapp F. Neurovirulence and induction of hydrocephalus with parental, mutant, and revertant strains of measles virus. J Virol. 1976 May;18(2):809–811. doi: 10.1128/jvi.18.2.809-811.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Huang A. S. Conserved peptides in the proteins of vesicular stomatitis virus. Virology. 1979 Jun;95(2):445–453. doi: 10.1016/0042-6822(79)90499-9. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dal Canto M. C., Rabinowitz S. G., Johnson T. C. An ultrastructural study of central nervous system disease produced by wild-type and temperature-sensitive mutants of vesicular stomatitis virus. Lab Invest. 1976 Aug;35(2):185–196. [PubMed] [Google Scholar]
- Dal Canto M. C., Rabinowitz S. G., Johnson T. C., Hughes J. V. Ultrastructural-immunohistochemical evidence for a maturation defect of temperature-sensitive G31 vesicular stomatitis virus in murine spinal cord neurons. Infect Immun. 1979 Apr;24(1):276–281. doi: 10.1128/iai.24.1.276-281.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto M. C., Rabinowitz S. G., Johnson T. C. Status spongiousus resulting from intracerebral infection of mice with temperature-sensitive mutants of vesicular stomatitis virus. Br J Exp Pathol. 1976 Jun;57(3):321–330. [PMC free article] [PubMed] [Google Scholar]
- Faulkner G., Dubois-Dalcq M., Hooghe-Peters E., McFarland H. F., Lazzarini R. A. Defective interfering particles modulate VSV infection of dissociated neuron cultures. Cell. 1979 Aug;17(4):979–991. doi: 10.1016/0092-8674(79)90337-4. [DOI] [PubMed] [Google Scholar]
- Fields B. N. Genetic manipulation of reovirus--a model for modification of disease. N Engl J Med. 1972 Nov 16;287(20):1026–1033. doi: 10.1056/NEJM197211162872007. [DOI] [PubMed] [Google Scholar]
- Haspel M. V., Duff R., Rapp F. Experimental measles encephalitis: a genetic analysis. Infect Immun. 1975 Oct;12(4):785–790. doi: 10.1128/iai.12.4.785-790.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haspel M. V., Lampert P. W., Oldstone M. B. Temperature-sensitive mutants of mouse hepatitis virus produce a high incidence of demyelination. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4033–4036. doi: 10.1073/pnas.75.8.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. V., Dille B. J., Thimmig R. L., Johnson T. C., Rabinowitz S. G., Dal Canto M. C. Neuroblastoma cell fusion by a temperature-sensitive mutant of vesicular stomatitis virus. J Virol. 1979 Jun;30(3):883–890. doi: 10.1128/jvi.30.3.883-890.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. V., Johnson T. C., Rabinowitz S. G., Dal Canto M. C. Growth and maturation of a vesicular stomatitis virus temperature-sensitive mutant and its central nervous system isolate. J Virol. 1979 Jan;29(1):312–321. doi: 10.1128/jvi.29.1.312-321.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D., Lodish H. F., Baltimore D. Analysis of the defects of temperature-sensitive mutants of vesicular stomatitis virus: intracellular degradation of specific viral proteins. J Virol. 1977 Mar;21(3):1140–1148. doi: 10.1128/jvi.21.3.1140-1148.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ngan J. S., Holloway A. F., Cormack D. V. Temperature-sensitive mutants of vesicular stomatitis virus: comparison of the in vitro RNA polymerase defects of group I and group IV mutants. J Virol. 1974 Oct;14(4):765–772. doi: 10.1128/jvi.14.4.765-772.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obijeski J. F., Simpson R. W. Conditional lethal mutants of vesicular stomatitis virus. II. Synthesis of virus-specific polypeptides in nonpermissive cells infected with "RNA-" host-restricted mutants. Virology. 1974 Feb;57(2):369–377. doi: 10.1016/0042-6822(74)90176-7. [DOI] [PubMed] [Google Scholar]
- Pringle C. R., Duncan I. B. Preliminary physiological characterization of temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1971 Jul;8(1):56–61. doi: 10.1128/jvi.8.1.56-61.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz S. G., Dal Canto M. C., Johnson T. C. Comparison of central nervous system disease produced by wild-type and temperature-sensitive mutants of vesicular stomatitis virus. Infect Immun. 1976 Apr;13(4):1242–1249. doi: 10.1128/iai.13.4.1242-1249.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond J. Y. Evidence for a mouse pathogenicity locus in certain temperature-sensitive mutants of foot-and-mouth disease virus. Infect Immun. 1977 Jun;16(3):827–831. doi: 10.1128/iai.16.3.827-831.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb J. A., Bond C. W., Leibowitz J. L. Pathogenic murine coronaviruses. III. Biological and biochemical characterization of temperature-sensitive mutants of JHMV. Virology. 1979 Apr 30;94(2):385–399. doi: 10.1016/0042-6822(79)90469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott R., Orlich M., Scholtissek C. Correlation of pathogenicity and gene constellation of influenza A viruses. III. Non-pathogenic recombinants derived from highly pathogenic parent strains. J Gen Virol. 1979 Aug;44(2):471–477. doi: 10.1099/0022-1317-44-2-471. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Koennecke I., Rott R. Host range recombinants of fowl plague (influenza A) virus. Virology. 1978 Nov;91(1):79–85. doi: 10.1016/0042-6822(78)90356-2. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Murphy B. R. Host range mutants of an influenza A virus. Arch Virol. 1978;58(4):323–333. doi: 10.1007/BF01317824. [DOI] [PubMed] [Google Scholar]
- Scholtissek C., Vallbracht A., Flehmig B., Rott R. Correlation of pathogenicity and gene constellation of influenza A viruses. II. Highly neurovirulent recombinants derived from non-neurovirulent or weakly neurovirulent parent virus strains. Virology. 1979 Jun;95(2):492–500. doi: 10.1016/0042-6822(79)90503-8. [DOI] [PubMed] [Google Scholar]
- Simpson R. W., Obijeski J. F. Conditional lethal mutants of vesicular stomatitis virus. I. Phenotypic characterization of single and double mutants exhibiting host restriction and temperature sensitivity. Virology. 1974 Feb;57(2):357–368. doi: 10.1016/0042-6822(74)90175-5. [DOI] [PubMed] [Google Scholar]
- Sonnenfeld G., Salvin S. B., Youngner J. S. Cellular source of interferons in the circulation of mice with delayed hypersensitivity. Infect Immun. 1977 Nov;18(2):283–290. doi: 10.1128/iai.18.2.283-290.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo J., Gibbs C. J., Jr, Gajdusek D. C., Toh B. H., Wurth M. Method for preparing cultures of central neurons: cytochemical and immunochemical studies. Proc Natl Acad Sci U S A. 1980 Jan;77(1):653–657. doi: 10.1073/pnas.77.1.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanners C. P., Goldberg V. J. On the mechanism of neurotropism of vesicular stomatitis virus in newborn hamsters. Studies with temperature-sensitive mutants. J Gen Virol. 1975 Dec;29(3):281–296. doi: 10.1099/0022-1317-29-3-281. [DOI] [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R., Macpherson T. M. Temperature-dependent host range mutation in vesicular stomatitis virus affecting polypeptide L. J Virol. 1977 May;22(2):381–388. doi: 10.1128/jvi.22.2.381-388.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilágyi J. F., Pringle C. R. Virion trascriptase activity differences in host range mutants of vesicular stomatitis virus. J Virol. 1975 Oct;16(4):927–936. doi: 10.1128/jvi.16.4.927-936.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr G. C., Armstrong J. A., Ho M. Production of interferon and serum hyporeactivity factor in mice infected with murine cytomegalovirus. Infect Immun. 1978 Mar;19(3):903–907. doi: 10.1128/iai.19.3.903-907.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R. Pathogenicity and immunogenicity for mice of temperature-sensitive mutants of vesicular stomatitis virus. Infect Immun. 1974 Aug;10(2):309–315. doi: 10.1128/iai.10.2.309-315.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H. L., Drayna D., Averill D. R., Jr, Fields B. N. Molecular basis of reovirus virulence: role of the S1 gene. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5744–5748. doi: 10.1073/pnas.74.12.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Dubovi E. J., Quagliana D. O., Kelly M., Preble O. T. Role of temperature-sensitive mutants in persistent infections initiated with vesicular stomatitis virus. J Virol. 1976 Jul;19(1):90–101. doi: 10.1128/jvi.19.1.90-101.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Quagliana D. O. Temperature-sensitive mutants of vesicular stomatitis virus are conditionally defective particles that interfere with and are rescued by wild-type virus. J Virol. 1976 Jul;19(1):102–107. doi: 10.1128/jvi.19.1.102-107.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]