Abstract
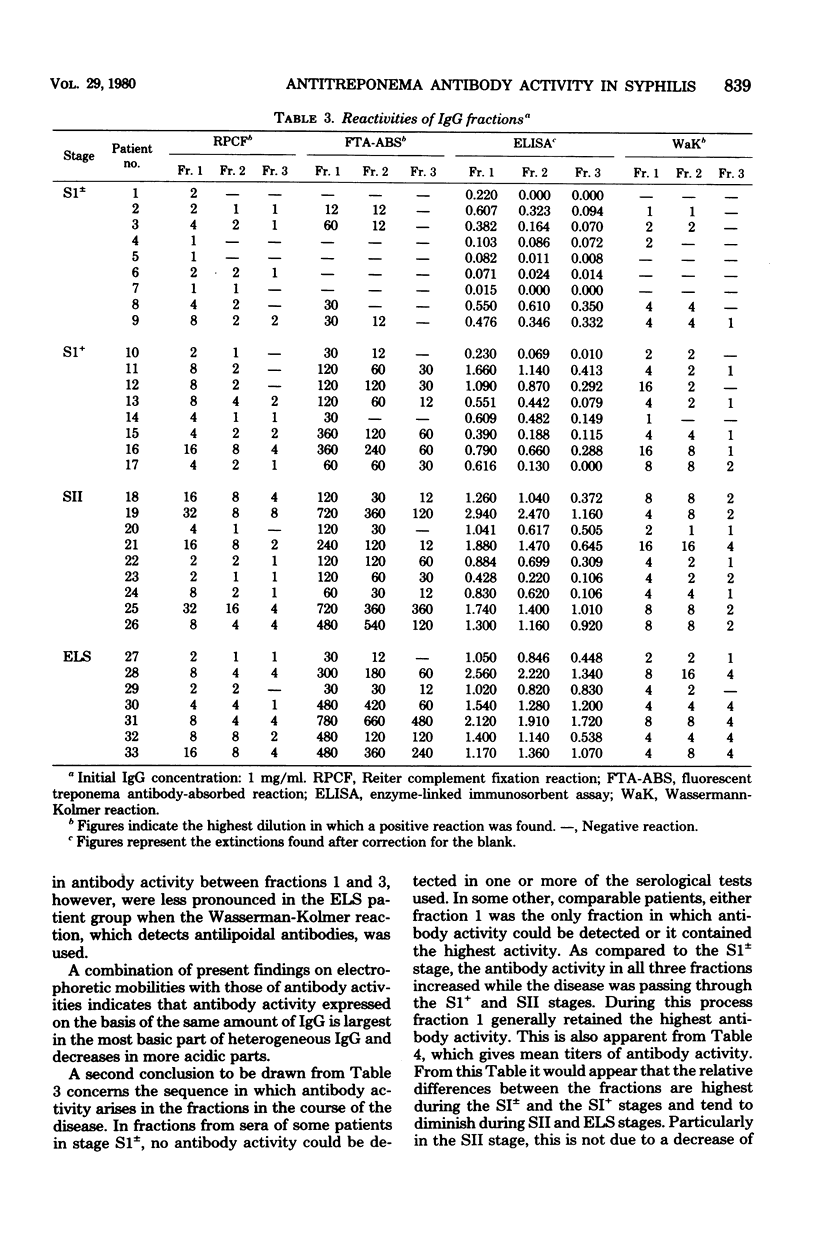
Three fractions, containing immunoglobulin G's (IgG's) of differing relative electrophoretic mobility, were isolated from sera of 33 male patients with untreated early syphilis. Serological testing, employing four different serodiagnostic procedures for these fractions at equal amounts of IgG, revealed a very constant reaction pattern for all patients. In the earliest stages of the disease, the most basic fraction was the first to show antibody activity. In progressive stages, antibody activity subsequently was also found in the two less basic fractions. However, in all stages of disease studied, the basic part of the IgG made the largest contribution of the total antibody activity within the IgG class, indicating an uneven distribution of antitreponema antibody activity over heterogeneous IgG. Several possible explanations of the observations are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baughn R. E., Musher D. M. Altered immune responsiveness associated with experimental syphilis in the rabbit: elevated IgM and depressed IgG responses to sheep erythrocytes. J Immunol. 1978 May;120(5):1691–1695. [PubMed] [Google Scholar]
- Delhanty J. J., Catterall R. D. Immunoglobulins in syphilis. Lancet. 1969 Nov 22;2(7630):1099–1103. doi: 10.1016/s0140-6736(69)90704-1. [DOI] [PubMed] [Google Scholar]
- Falkenberg F. W., Karniely Y., Schmitt-Verhulst A. M., Mozes E., Sela M. Lack of inverse relationship between the net charge of T-independent immunogens and the responding spleen cells. J Immunol. 1975 Dec;115(6):1538–1541. [PubMed] [Google Scholar]
- Levene G. M., Turk J. L., Wright D. J., Grimble A. G. Reduced lymphocyte transformation due to a plasma factor in patients with active syphilis. Lancet. 1969 Aug 2;2(7614):246–247. doi: 10.1016/s0140-6736(69)90010-5. [DOI] [PubMed] [Google Scholar]
- Levene G. M., Wright D. J., Turk J. L. Cell-mediated immunity and lymphocyte transformation in syphilis. Proc R Soc Med. 1971 Apr;64(4):426–428. [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Baseman J. B., Folds J. D. Selective response of lymphocytes from Treponema pallidum-infected rabbits to mitogens and Treponema reiteri. Infect Immun. 1977 Feb;15(2):417–422. doi: 10.1128/iai.15.2.417-422.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavia C. S., Folds J. D., Baseman J. B. Depression of lymphocyte response to concanavalin A in rabbits infected with Treponema pallidum (Nichols strain). Infect Immun. 1976 Jul;14(1):320–322. doi: 10.1128/iai.14.1.320-322.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavis C. S., Folds J. D., Baseman J. B. Cell-mediated immunity during syphilis. Br J Vener Dis. 1978 Jun;54(3):144–150. doi: 10.1136/sti.54.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puritz E. M., Thompson J. A., Jr, Dierberg F., Kraus S. J., Yount W. J. IgG subclasses of fluorescent treponemal antibodies: correlation with complement fixation and clinical stage. Clin Immunol Immunopathol. 1975 Sep;4(3):352–361. doi: 10.1016/0090-1229(75)90004-5. [DOI] [PubMed] [Google Scholar]
- Sela M., Mozes E. Dependence of the chemical nature of antibodies on the net electrical charge of antigens. Proc Natl Acad Sci U S A. 1966 Feb;55(2):445–452. doi: 10.1073/pnas.55.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underdown B. J., Goodfriend L. Correlation of charge properties of human reaginic antibodies with charge of the corresponding allergens. J Immunol. 1970 Feb;104(2):530–533. [PubMed] [Google Scholar]
- Van Der Sluis J. J., Menke H. E., Neumann H. Preference for basic IgG in early syphilis. Br J Vener Dis. 1975 Jun;51(3):161–164. doi: 10.1136/sti.51.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Sluis J. J., Menke H. E. Role of IgG fractions with high isoelectric points in the thymol turbidity test in syphilis. Evidence for an increase in basic IgG in early syphilis. Br J Vener Dis. 1975 Jun;51(3):158–160. doi: 10.1136/sti.51.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldkamp J., Visser A. M. Application of the enzyme-linked immunosorbent assay (ELISA) in the serodiagnosis of syphilis. Br J Vener Dis. 1975 Aug;51(4):227–231. doi: 10.1136/sti.51.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J. L., Folds J. D., Baseman J. B. Serum of rabbits infected with Treponema pallidum (Nichols) inhibits in vitro transformation of normal rabbit lymphocytes. Cell Immunol. 1979 Feb;42(2):363–372. doi: 10.1016/0008-8749(79)90201-6. [DOI] [PubMed] [Google Scholar]
- Wicher V., Wicher K. In vitro cell response of Treponema pallidum-infected rabbits. I. Lymphocyte transformation. Clin Exp Immunol. 1977 Sep;29(3):480–486. [PMC free article] [PubMed] [Google Scholar]
- de Jong N. H., van der Sluis J. J., van Dijk J., Feltkamp T. E. Epidermal antibodies in secondary syphilis. Br J Vener Dis. 1978 Aug;54(4):283–283. doi: 10.1136/sti.54.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]


