Figure 3.
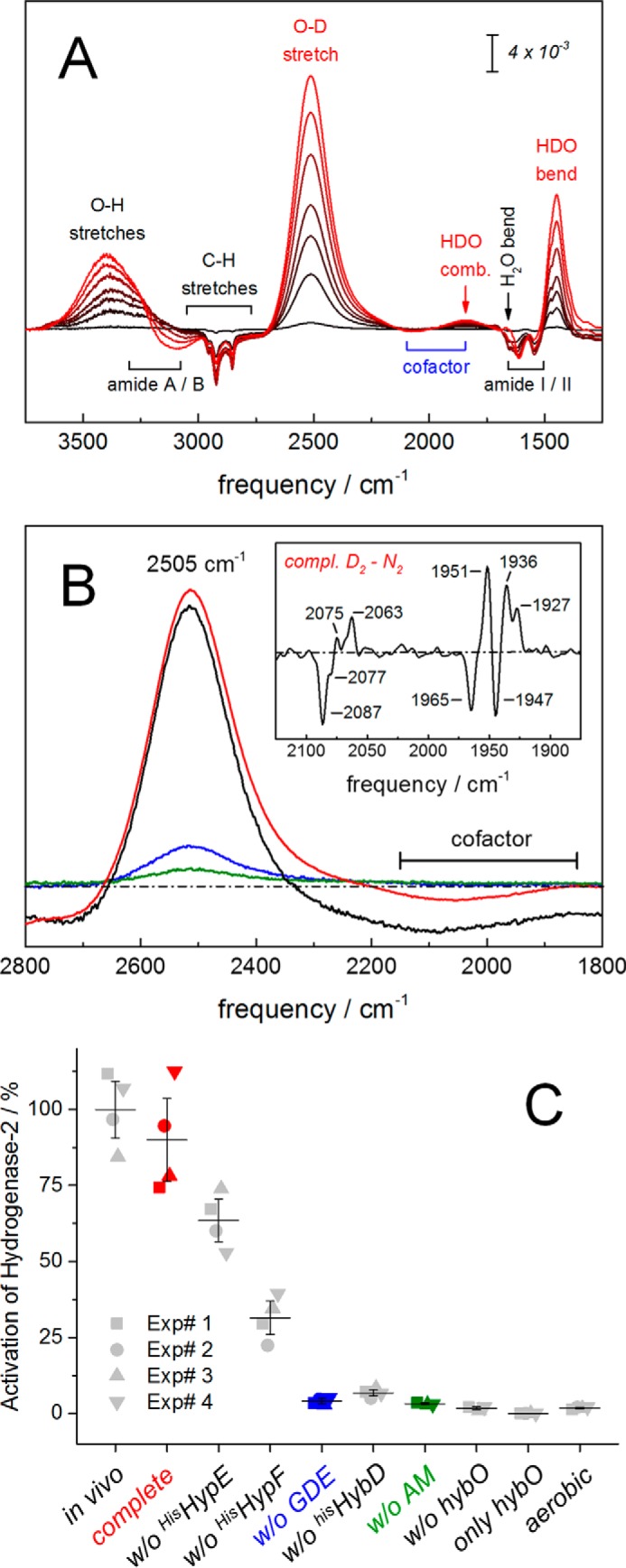
Analysis of hydrogenase activity matured in vitro. A, FTIR analysis of oxidation activity of in vitro maturated hydrogenase-2 in the presence of H2O and D2. Catalytic activity is followed via D2 oxidation and release of deuterium ions into the H2O bulk. This induces a hydrogenase-specific increase of HDO (bands at 2505, 1850, and 1445 cm−1; red labels). Further signals show a slight increase of liquid water (O–H stretches at 3400 cm−1 and H2O bending around 1660 cm−1) and a concomitant decrease of protein bands (i.e. CH stretches from 3000 to 2750 cm−1, amide I/II vibrations from 1650 to 1500 cm−1, and a decrease of N–H stretches from 3300 to 3100 cm−1 (amide A/B) (60). B, matured hydrogenase-2 as isolated (in vivo, black) or as synthesized in vitro (in vitro complete, red) did not differ significantly in D2 uptake activity. Residual activity was observed in the absence of the HybG–HypDE complex (in vitro w/o GDE, blue) or activation mixture (in vitro w/o AM, green). Note the D2-N2 difference spectrum of in vitro synthesized hydrogenase-2 (inset). Here, the population of at least three reduced species is shown (positive CO frequencies at 1951, 1936, and 1927 cm−1). C, kinetic analysis of H2 uptake activity. The complete assay was composed of purified HybC, GDE, HypE, HypF, the endopeptidase HybD, HybO, and AM. Reactions were conducted in the absence of one or more substrates at a time, as indicated. The samples were analyzed for activity by following H2-dependent reduction of benzyl viologen photometrically under the same reaction conditions. The specific activity of purified hydrogenase-2 (in vivo) was 1.88 μmol of H2 oxidized/min/mg. This activity represents 100% activation and provides an estimate of the maximum activity expected for the in vitro matured hydrogenase. The maximal attainable activity obtained under this condition was in the presence of all components of the in vitro assay (complete). Without the addition of an equimolar ratio of HisHypE and HisHypF to the reaction mix, ∼70 or 30% of enzyme activity could be restored, respectively. Only background activity was observed when GDE, HybD, AM, or HybO was omitted or when the incubation was performed under aerobic conditions. HybO alone shows no hydrogenase activity. Four independent experiments and the S.D. value is shown.
