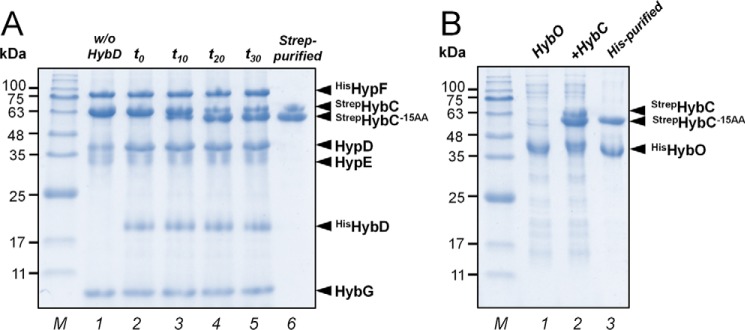
Figure 5.
SDS-PAGE analysis of protein compositions of in vitro assays at different maturation steps. A, in vitro processing of hydrogenase-2 catalytic subunit HybC. Lane 1, reaction mix contained HybC, GDE complex, HypE, and HypF in stoichiometric amounts (2 μm each) and AM. Lane 2, the reaction was started by the addition of endopeptidase HybD in catalytic amounts, and immediately an aliquot of the reaction mix (∼25 μg) was analyzed (t0). No HybC−15AA was detected. Lanes 3–5, aliquots of the reaction mix were analyzed after 10, 20, and 30 min of incubation at room temperature, respectively (t10, t20, and t30). HybC−15AA formation was observed over time. After 20 min, no additional processing of HybC was observed. Lane 6, isolation of both unprocessed StrepHybC and processed StrepHybC−15AA from the reaction mix by Strep-Tactin affinity chromatography. B, formation of HybC–HybO holoenzyme. Lane 1, HisHybO-enriched fraction. Lane 2, the StrepHybC and StrepHybC−15AA fraction isolated from reaction mix was supplemented with the HisHybO-enriched fraction. Lane 3, the StrepHybC–HisHybO heterocomplex was isolated by a histidine affinity column. Only the processed large subunit (HybC−15AA) can form a heterodimer with the small subunit HybO.