Figure 5.
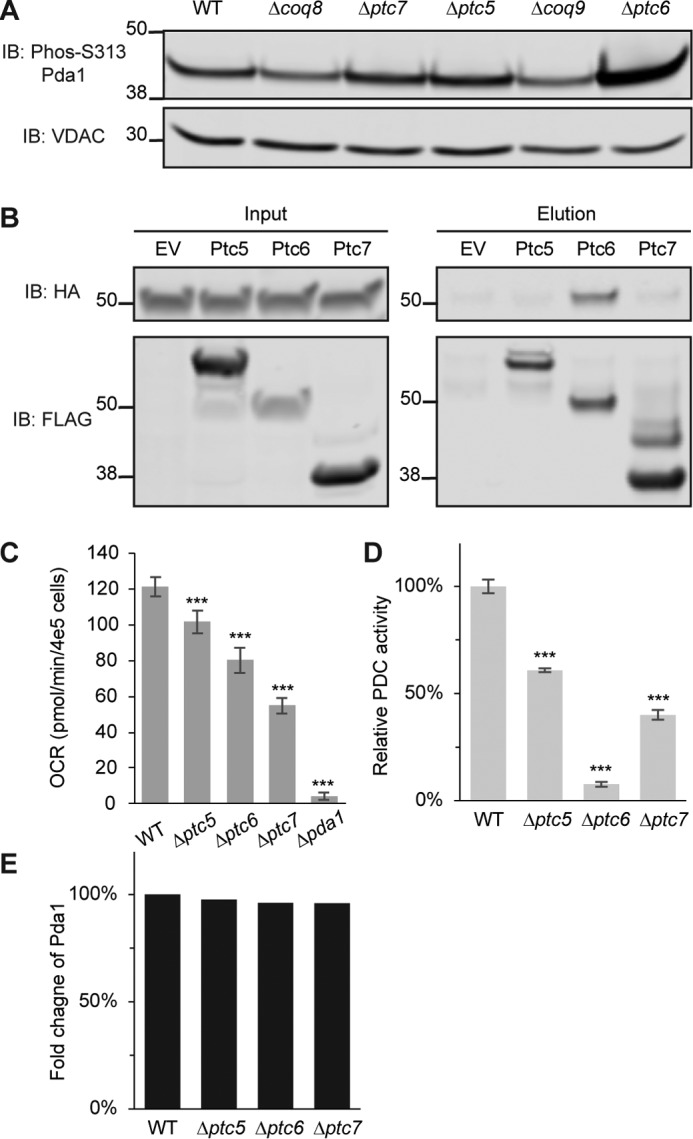
In vitro biochemical assays verify Ptc6p as a Pda1p phosphatase. A, immunoblot (IB) of phosphorylated Pda1p on Ser-313 or voltage-dependent anion channel (VDAC) (loading control). The samples are mitochondrial lysates from WT, Δcoq8, Δptc7, Δptc5, Δcoq9, and Δptc6 yeast. B, immunoblot of HA (Pda1p was endogenously tagged with HA) or FLAG (bait proteins were C-terminally FLAG-tagged). Samples in the left panels are input lysates for the anti-FLAG immunoprecipitations from WT yeast strains overexpressing empty vector (EV), Ptc5p, Ptc6p, and Ptc7p. Samples in the right panels are the corresponding eluates from the same immunoprecipitations. C, oxygen consumption rates (OCR) of WT, Δptc5, Δptc6, Δptc7, and Δpda1 in synthetic complete medium containing 2% pyruvate (mean ± S.D., n = 6). D, relative PDC activity of mitochondrial lysates from WT, Δptc5, Δptc6, and Δptc7 (mean ± S.D., n = 3). E, fold change of Pda1p protein quantified by MS from same samples in D (normalized to WT). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
