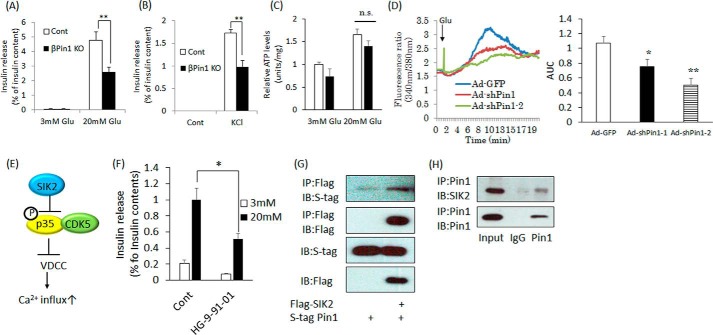
Figure 5.
Ablation of the Pin1 gene impairs the insulin release response. A and B, measurement of insulin release in isolated islets stimulated with 20 mm glucose for 1 h or with 45 mm KCl for 30 min. HKR buffer containing 3 mm glucose was used as a control. Supernatants were collected, and insulin contents were measured by the ELISA method. The rates of insulin release were calculated by adjusting for total insulin contents (n = 4–5). C, measurement of intracellular ATP levels in the isolated islets. Isolated islets were stimulated with 3 or 20 mm glucose for 15 min. Then ATP levels were measured. D, Min6 cells were loaded with Fura-2 for 30 min. After being washed with PBS, intracellular Ca2+ changes were measured. High glucose was added 1 min after starting the measurement (n ≥ 25 cells). E, regulation of insulin release by SIK2. F, decreased insulin release in response to pan-SIK inhibitor treatment. Isolated islets were incubated with 1 μm HG-9-91-01 overnight and then stimulated with 20 mm glucose for 1 h (n = 4–5). G, the association between Pin1 and SIK2. Both FLAG-SIK2 and S-tagged Pin1 were overexpressed in 293T cells. Next, the cells were immunoprecipitated (IP) with FLAG beads, and binding proteins were eluted employing FLAG peptide. H, Pin1 endogenously binds to SIK2. Proteins were extracted from Min6 cells and then immunoprecipitated with Pin1 antibody. Finally, proteins in the samples were detected employing SIK2 antibody. *, p < 0.05; **, p < 0.01; n.s., not significant. Representative data from three independent experiments are shown. IB, immunoblotting; AUC, area under the curve; error bars, S.E.