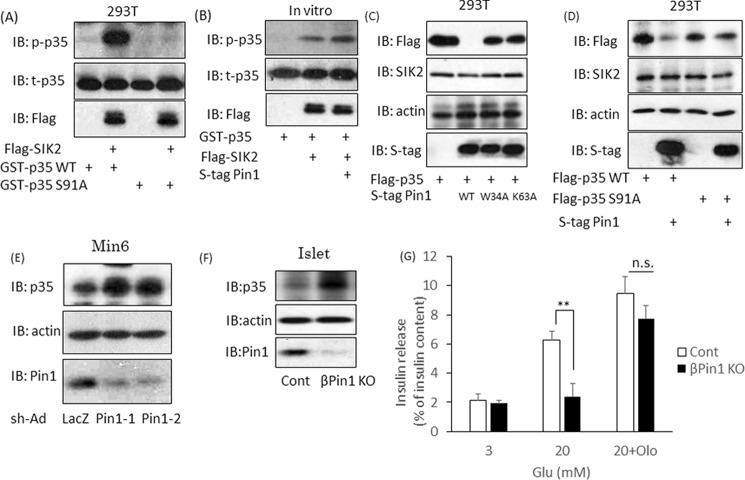
Figure 7.
SIK2 is activated by the association with Pin1 and is involved in the insulin secretion pathway. A, phosphorylation of p35 at Ser-91 by SIK2. SIK2 protein was incubated with GST–p35 WT or GST–p35 S91A for 30 min, and the level of phospho-p35 was then examined by Western blotting (IB). B, up-regulation of SIK2 activity by Pin1. GST–p35 protein was reacted with SIK2, and SIK2 binding with Pin1 was allowed to proceed for 30 min. Phosphorylation levels of p35 were determined by Western blotting. C, Pin1 decreases p35 expression, depending on the isomerase activity of Pin1. Both FLAG-p35 and S-tag Pin1 were transfected into 293T cells. The p35 expression levels were determined by Western blotting. W34A is the Pin1 mutant form that is unable to bind substrates, and K63A is the inactive form of PPIase. D, the reductions in p35 proteins in response to Pin1 depend on SIK2 activity. Both S-tag Pin1 and FLAG-SIK2 were transfected into 293T cells. The SIK2 S91A mutant is not phosphorylated by SIK2. E, Min6 cells were infected with adenovirus coding shLacZ or shPin1. After 72 h, samples were prepared, and total p35 levels were determined by Western blotting. F, islets were isolated from both control and βPin1 KO mice. The p35 protein expression levels were investigated using Western blotting. G, isolated islets were incubated with DMSO or 50 μm olomoucine for 2 h. The cells were then incubated in the presence of 20 mm glucose for 1 h (n = 4–5). **, p < 0.01; n.s., not significant. Shown are representative data from three independent experiments. Error bars, S.E.