Abstract
An LC-MS/MS method to measure ribociclib in mouse plasma and Ringer’s solution was successfully developed and validated. Reverse phase chromatography was performed with gradient elution using C18 (100A, 50x4.6 mm, 3μ) and C8-A (50x 2.0 mm, 5 μ) columns for plasma and Ringer’s samples, respectively. Mouse plasma samples were extracted using solid phase extraction method, whereas no extraction was required for the Ringer’s solution samples. Analytes were detected using positive ion MRM mode. The precursor to product ions (Q1→Q3) selected for ribociclib and d6-ribociclib were (m/z) 435.2 → 252.1 and 441.2 → 252.1, respectively. The linear range of quantification of ribociclib was 62.5–10000 ng/ml for plasma method and 0.1–100 ng/ml for Ringer’s solution method. The results for the inter-day and intra-day accuracy and precision of quality control samples were within the acceptable range. The lower limit of quantitation (LLOQ) for plasma and Ringer’s samples were 62.5 ng/ml (S/N > 30) and 0.1 ng/ml (S/N > 13), respectively, whereas the limit of detection (LOD) was 6.9 ng/ml (S/N > 7) and 0.05 ng/ml (S/N > 3), respectively. The developed methods were successfully applied to the analysis of ribociclib in mouse plasma and dialysate samples collected during a cerebral microdialysis study of ribociclib in a non-tumor bearing mouse.
Keywords: Ribociclib, CDK4/6, LC-MS/MS, Solid Phase extraction, Microdialysis, Ringer’s solution
1. Introduction
Primary tumors of the central nervous system (CNS) remain a significant cause of death in children. According to the Central Brain Tumor Registry of the United States (CBTRUS), cancer is one of the leading causes of death in children age 0–14 and brain tumors are the most common cause of cancer death [1]. Less than 10% of children with malignant brain tumors that have recurred after initial conventional therapy survive. Thus, it is essential that novel approaches to therapy be developed and implemented.
In that regard, enhanced molecular interrogation of pediatric CNS tumors by transcriptomic, methylomic, and mutational profiling has uniformly revealed that these are highly heterogeneous diseases even when grouped by histologic diagnosis [2–4]. Molecular interrogation, by way of generating comprehensive data on disease entities, has yielded new and exciting therapeutic leads for pediatric brain tumors.
Ribociclib, an example of such a therapeutic class of compounds, is an inhibitor of CDK4/6, which are cyclin D-dependent kinases that are instrumental mediators of cell cycle progression from G1 to S phase [5, 6]. Although this compound is currently under clinical evaluation by Novartis for treatment of many solid tumors including pediatric brain tumors (clinicaltrials.gov NCT02607124), nothing is known about the CNS penetration of this compound. Thus, as a first step toward gaining a better understanding of the CNS penetration, we desired to perform CNS microdialysis studies. This required that we have a sensitive and specific LC-MS/MS method for ribociclib in mouse plasma and Ringer’s solution. Although others have published on the murine pharmacokinetics of ribociclib [7], to our knowledge no LC-MS/MS method for ribociclib has been published. Thus, we developed LC-MS/MS methods for mouse plasma and Ringer’s solution that we could use in our cerebral microdialysis experiments, which will be used intensively to study the CNS penetration of ribociclib in non-tumor bearing mice.
2. Materials and methods
2.1 Reagents and chemicals
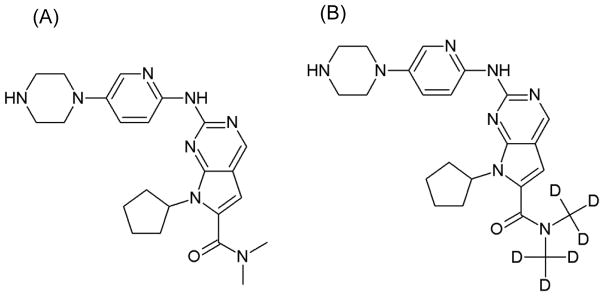
Ribociclib succinate (Fig. 1) and deuterated ribociclib (d6-ribocliclib, Fig. 1) were supplied by Novartis (Basel, Switzerland). Ammonium acetate, methanol, 85% o-phosphoric acid and LC-MS/MS grade water were purchased from Fisher Scientific (Fairlawn, NJ, USA). Acetonitrile was obtained from Honeywell Burdick & Jackson (Morris Plains, NJ, USA) and formic acid from Sigma (St. Louis, MO, USA). Ringer’s solution, an isotonic solution of sodium chloride, potassium chloride, calcium chloride, and sodium bicarbonate (to balance the pH) dissolved in water, was purchased from Frey Scientific (Nashua, NH, USA). Mouse (CD-1) plasma sodium heparin was obtained from BioreclamationIVT (NY, USA).
Figure 1.
Structure of (A) ribociclib and (B) internal standard, d6-ribociclib.
2.2 Stock Solutions
Stock solutions were prepared by dissolving accurately weighted ribociclib or d6-ribociclob in a solution consisting of 50/50 methanol/water (v/v) to yield a concentration of 1.00 mg/ml.
Ribociclib stock solution was further diluted in 50/50 methanol/water (v/v) to prepare working solutions for calibration curve and quality control samples. Working solutions for plasma calibration curve and quality control (QC) samples ranged from 125 to 20000 ng/mL, whereas that for Ringer’s samples ranged from 0.500 to 500 ng/mL.
Internal standard (ISTD) working solution was prepared from 1.00 mg/ml stock solution using the same solvent to a concentration of 800 ng/ml for plasma samples and 25.0 ng/ml for Ringer’s solution samples.
2.3 Calibration curve and quality controls
Plasma calibrators were prepared by adding 10 μl of ribociclib working solution and 10 μl of ISTD working solution to 20 μl of blank mouse (CD-1) plasma to yield concentrations of 62.5, 125, 250, 500, 1000, 2000, 5000, and 10000 ng/ml. Ringer’s solution calibrators were prepared by adding 5 μl of ribociclib working solution and 5 μl of ISTD working solution in 25 μl of blank Ringer’s solution to give final concentrations of 0.100, 0.500, 0.750, 1.00, 5.00, 10.0, 25.0, 50.0, and 100 ng/ml. LLOQ (lower limit of quantitation), LQC (low QC), MQC (medium QC) and HQC (High QC) samples were prepared at concentrations of 62.5, 187.5, 650, and 7500 ng/ml for plasma samples and 0.100, 0.900, 40.0, and 75.0 ng/ml for Ringer’s samples.
2.4 Sample preparation
2.4.1 Mouse plasma sample preparation
A solid phase extraction technique was used to extract the analytes from the plasma samples. To 20 μl of unknown plasma samples in a siliconized tube (Fisherbrand, Fairlawn, NJ, USA), 10 μl of 50/50 methanol/water (v/v) and 10 μl of ISTD working solution were added. The above solution was then acidified by addition of 100 μl of 28% phosphoric acid, followed by vortexing for 30 sec. Waters (Milford, MA, USA) Oasis30 μm HLB 96-well microelution plate was pre-conditioned first with 200 μl of methanol and then with 200 μl of water under gentle vacuum. Acidified samples were transferred into appropriate wells and then passed through the wells under gentle vacuum. Wells were washed twice with 200 μl of water and then analytes were eluted with 100 μl of methanol into a clean 96-well plate. The eluted solution was diluted with 100 μl of water, and 3 μl was injected for analysis on the LC-MS/MS system.
2.4.2 Ringer’s Sample preparation
No extraction was required to prepare the Ringer’s samples. Briefly, 25 μl of unknown Ringer’s solution sample was spiked with 5 μl of ISTD working solution and 5 μl of 50/50 methanol/water (v/v) in a siliconized tube. 5 μl of 50/50 methanol/water (v/v) was added in order to make up the same volume and proportion of organic to aqueous phase as used in the preparation of calibrators and quality controls. All samples were acidified using 75 μl of 0.2% formic acid in water. Samples were then vortexed and centrifuged for 30 sec. All samples were then transferred into autosampler vials and 6 μl was injected for analysis on the LC-MS/MS system.
2.5 Chromatographic conditions
The HPLC system for the plasma method consisted of a Shimadzu (Kyoto, Japan) system controller (CBM-20A), pumps (LC-20AD), autoinjector (SIL-20ACHT), online degasser (DGU-14A), and Phenomenex (Torrance, CA, USA) HPLC column heater (Thermasphere TS-130). Separation of analytes was performed using a Phenomenex Luna (Torrance, CA, USA) C18 column (100A, 50x4.6 mm, 3μ) at 50 ºC. A gradient HPLC was used to elute analytes of interest. Elution was done at a flow rate of 1 ml/min. Mobile phase-A contained 20 mM ammonium acetate in water and mobile phase-B contained 20 mM ammonium acetate in methanol. A needle wash solution of 50/50 methanol/water (v/v) was used. The gradient starting conditions were set at 70% mobile phase A and 30% mobile phase B, and this was kept constant for an initial 0.30 min. The percentage of mobile phase B was then changed from 30% to 98% from 0.30 min to 3.00 min, maintained at 98% until 4.30 min, decreased to 30% until 4.50 min and then maintained at 30% until 5.00 min.
The HPLC system for the Ringer’s solution method consisted of a Shimadzu system controller (CBM-20A), pumps (LC-20AD XR), autoinjector (SIL-20ACXR), online degasser (DGU-20A3), and column oven (CTO-20AC). An Agilent Polaris (Santa Clara, CA, USA) C8-A column (50x 2.0 mm, 5 μ) was used for the separation. Flow rate was set to 0.6 ml/min and analysis was performed at 25 ºC. Mobile phase-A consisted of 0.1% formic acid in water and mobile phase-B consisted of 0.1% formic acid in acetonitrile. The needle wash solution used was 0.1% formic acid in 50/50 methanol/water (v/v). The starting condition of the gradient was set at 5% mobile phase B and was kept at 5% for initial 0.4 min. The percentage mobile phase B was then increased from 5% to 99% from 0.40 min to 2.20 min, maintained at 99% until 3.20 min, decreased to 5% until 5.00 min and then maintained at 5% until 5.50 min.
2.6 Mass spectrometric conditions
An AB SCIEX QTRAP 4000 and QTRAP 5500 mass spectrometers equipped with a Turbo IonSpray source were used for the detection of analytes in mouse plasma and Ringer’s solution, respectively. The instruments were operated using Analyst software (Version 1.6.2, AB SCIEX). Positive ion multiple reaction monitoring (MRM) mode was employed for the analysis. The precursor to product ions (Q1→Q3) selected for ribociclib and d6-ribociclib were (m/z) 435.2 → 252.1 and 441.2 → 252.1 respectively.
The optimized MS/MS conditions for the QTRAP 4000 were: curtain (CUR) gas pressure set at 10 psi, collision activated dissociation (CAD) at medium, ionspray voltage (IS) at 5500V, temperature (TEM) at 600 ºC, gas 1 (GS1) pressure at 20 psi, gas 2 (GS2) pressure at 40.0 psi, interface heater (ihe) set to ON, declustering potential (DP) at 125.0 V, entrance potential (EP) at 10.0V, collision energy (CE) at 68.0V, and collision exit potential (CXP) at 14.0 V. The optimized MS/MS conditions for the QTRAP 5500 were: CUR gas pressure set at 25 psi, collision CAD at medium, IS at 2500 V, TEM at 650 ºC, GS1 pressure at 70.0 psi, GS2 pressure at 70.0 psi, DP at 61.0 V, EP at 11.0 V, CE at 63.0 V, and CXP at 22.0 V.
2.7 Method validation
2.7.1 Linearity
The Ringer’s solution method was developed first and underwent a full 5-day validation. The plasma method had minor changes to the method from the Ringer’s method, and thus underwent a partial validation that was performed over three days. To establish method linearity, a calibration curve was prepared in the appropriate matrices and using the method of 1/x2 weighted least squares regression, different parameters were calculated including slope, intercept and correlation coefficient of each calibration curve.
2.7.2 Precision and accuracy
The precision and accuracy of the assay were determined on a single day (intra-day) and on different days (inter-day). Six replicates were prepared at the assay LLOQ (lower limit of quantitation), LQC (low QC), MQC (medium QC) and HQC (High QC) concentrations. Inter-assay and intra-assay precision and accuracy were expressed as the percentage coefficient of variance (%CV) and percentage relative error (%RE), respectively.
2.7.3 Lower limit of quantification and limit of detection (LOD)
The LLOQ was defined as the lowest analyte concentration in the calibration curve that could be determined with acceptable precision (i.e., CV less than 20%) and accuracy (i.e., within 20% of the nominal value) and with a signal to noise ratio of at least 5. The LOD was defined as the lowest analyte concentration having a signal to noise ratio of at least 3.
2.7.4 Selectivity, carry-over, and matrix effect
The selectivity study was performed to confirm that this newly developed method could successfully separate ribociclib and ISTD from potentially interfering components in the matrix of interest. Selectivity was assessed by blank samples and spiked samples extracted at the assay LLOQ in three different mouse plasma sources. In the case of Ringer’s solution selectivity study, only one source was incorporated. Carry-over was assessed by injecting wash samples consisting of 0.2% formic acid after samples with expected high concentrations (e.g., ULQ and HQC). The matrix effect was defined as the degree to which elements in the sample matrix affected the detection of the analyte of interest.
The matrix effect was assessed by spiking an extracted blank and neat sample with ribociclib and ISTD at the assay LQC and HQC (n=3 samples each) in three different mouse plasma sources (CD1, CD1 nude and FVB mouse plasma). The matrix factor was quantitated by taking the peak area ratio of the chromatographic response in spiked blank extracted samples to that in neat samples. The ISTD normalized matrix factor was also calculated. It was calculated by taking the ratio of ribociclib matrix factor to ISTD matrix factor of the same source [8].
2.7.5 Stability
Stability was defined as the measure of the ability of the analyte to preserve its chemical properties in a matrix. To access ribociclib stability over time and at different temperatures in plasma and Ringer’s solution, LQC and HQC samples were prepared in triplicates for each condition. Stability in mouse plasma was evaluated at 4ºC, room temperature, 37ºC for up to 24 h, and at −80ºC for up to 163 days. Stability in Ringer’s solution was tested at 4ºC, room temperature, and 37ºC for up to 24 h, and −80 ºC for up to 40 days. Stability was reported in terms of % change in the concentration at the time of stability study with respect to the concentration at time 0. Sample was considered stable when the % change in the concentration was within ±15%. For extract stability study, extracted LQC and HQC samples were stored at 4 ºC in the autosampler after analysis and reinjected after 24 h (Ringer’s sample) and 48 h (plasma samples).
The stock solution stability study was performed by preparing a master stock solution and storing aliquots at −80ºC for up to 71 days. Both the old and fresh master stock solutions were diluted to equal concentration, and then equal concentration of ISTD was added. Stability was calculated by comparing the average area ratio of the old master stock to the fresh master stock determined from replicate injections (n=6).
2.8 Cerebral microdialysis study
The cerebral microdialysis study was performed using a non-tumor bearing female CD1 nude mouse (Charles River, Wilmington, MA) with an IACUC-approved procedure described previously [9, 10]. A precalibrated microdialysis probe with 38 MWCO membrane (MD-2211, BASi) was inserted into the guide cannula implanted in the cortex. The microdialysis probe was perfused with Ringer’s solution at a flow rate of 0.5 μL/min. After equilibration with the in vivo environment for an hour, the mouse was dosed with 100 mg/kg ribociclib via oral administration. The dosing solution was formulated by dissolving an appropriate amount of ribociclib in 0.5% methylcellulose (Sigma-Aldrich, St. Louis, Mo) to prepare a concentration of 10 mg/mL. After dosing, microdialysis fractions were collected over 1 h intervals up to 7 h. Blood samples from the mouse were collected at 5 min and 1.5 and 7 h post-dose using an eye-bleed technique. Immediately after collection, blood samples were centrifuged and plasma was separated. Plasma samples were stored at 80 °C until further analysis, whereas dialysate samples were immediately analyzed at the end of experiment. Microdialysis probe recovery was calculated using in vitro recovery study using 1 μg/mL ribociclib solution prepared in Ringer’s solution.
3. Results and discussion
3.1 Chromatography and mass spectrometric conditions
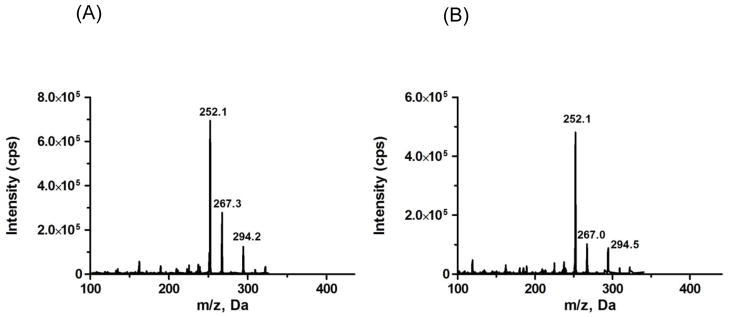
Based on the present functional groups on ribociclib and ISTD (Fig. 1), positive ion electrospray (MH+) was selected as the mode of ionization. Strong signal intensity was observed under this mode. Different fragments of ribociclib and ISTD were observed in product ion scan, but m/z 252.1 was selected for quantitation of both ribociclib and ISTD since it was the most abundant fragment ion. Fig. 2 shows the product ion scan of ribociclib and ISTD.
Figure 2.
Product ion scans for (A) ribociclib and (B) internal standard, d6-ribociclib.
The range of the ribociclib concentrations in the dialysate samples was expected to be lower than in the plasma samples. The expected LLOQ for the Ringer’s samples was 0.100 ng/ml, and since the LOD for the QTRAP 4000 was only 6.90 ng/ml (S/N >7), it was not expected that the QTRAP 4000 would have the necessary sensitivity. Therefore, it was essential to develop a highly sensitive method for application to the Ringer’s solution. To achieve this, a more sensitive mass spectrometer (i.e., QTRAP 5500) was utilized, in addition to a different column (i.e., Polaris C8-A column) and mobile phases. Using a different instrument and parameters, we were able to attain a LLOQ of 0.100 ng/ml and LOD of 0.0500 ng/ml in Ringer’s solution. However, sample carry-over was observed in samples injected after Ringer’s solution with high ribociclib concentrations (e.g., 75.0 to 100 ng/ml). To minimize this sample carry-over, we made several changes in the Ringer’s ribociclib method conditions to address the carry-over. As noted above, the HPLC column was changed from a Luna C18 to the Polaris C8-A. We observed a lower sensitivity with the C18 column, and postulated that one of the reasons might be the strong hydrophobic interaction of ribociclib with the column, which might lead to carry-over. We hypothesized that by reducing the hydrophobicity of the column from C18 to a polar modified C8 (C8-A) would help in reducing the strength of this hydrophobic interaction, thus reducing the carry-over. This in turn would result in better S/N ratio. When we used the C8-A column, we noted an increased sensitivity and sharper peaks. Since we still observed some carry-over, we employed a needle wash solution of 0.1% formic acid in 50/50 methanol/water (v/v). Additionally, to further minimize the potential for carry-over we made wash injections of 0.2% formic acid approximately every fifth injection. The combination of these approaches minimized the problem of carry-over (see Supplementary Material, Figs. S1 and S2).
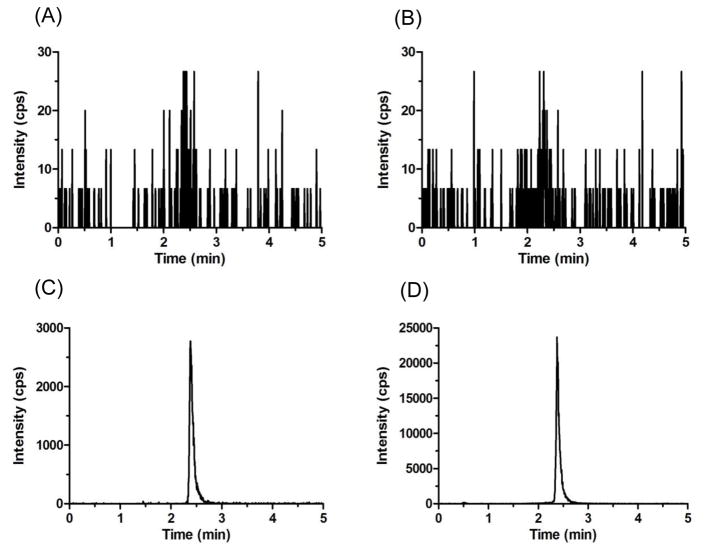
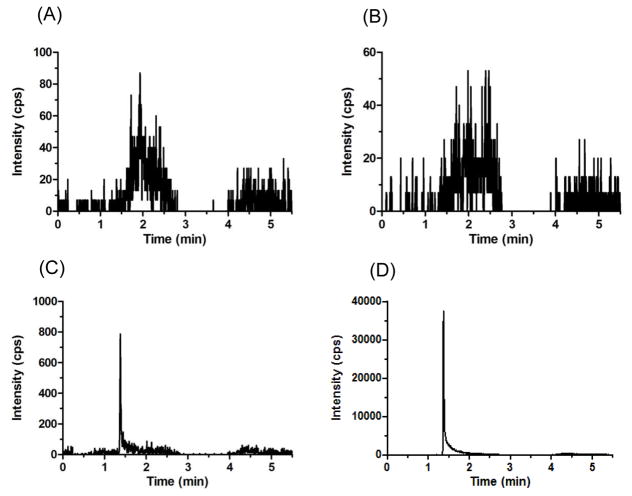
The retention time was 2.4 min for ribociclib and d6-ribociclib in mouse plasma (Fig. 3), and 1.4 min for ribociclib and d6-ribociclib in Ringer’s solution (Fig. 4).
Figure 3.
Representative MRM chromatograms of (A) ribociclib scan in blank mouse plasma, (B) ISTD scan in blank mouse plasma, (C) ribociclib scan in spiked LLOQ mouse plasma sample, and (D) ISTD scan in spiked LLOQ mouse plasma sample.
Figure 4.
Representative MRM chromatograms of (A) ribociclib scan in blank Ringer’s solution, (B) ISTD scan in blank Ringer’s solution, (C) ribociclib scan in spiked LLOQ Ringer’s solution sample, and (D) ISTD scan in spiked LLOQ Ringer’s solution sample.
3.2 Sample preparation
A solid phase extraction (SPE) technique was used for mouse plasma sample preparation. We chose this extraction technique because in our experience the SPE had minimal sample volume requirements, results were very reproducible, and the extract had minimal interference with the analyte of interest. Oasis HLB microelution plate 30 μm (Waters) was selected for solid phase extraction. The procedure was performed as described previously in Section 2.4.1. No extraction was required for preparation of the Ringer’s solution sample. Samples were simply acidified, and diluted with 0.2% formic acid in water.
3.3 Linearity
The assay was linear over the concentration range of 62.5–10000 ng/ml in plasma and 0.100–100 ng/ml in Ringer’s solution. The correlation coefficients (R2) were greater than 0.996 and 0.990 for plasma and Ringer’s solution calibration curves, respectively. In addition, the mean (% CV) value of slopes were less than 0.003 (1.69%) and 0.220 (2.45%) for plasma and Ringer’s solution calibration curves, respectively
3.4 Precision and accuracy
The results for the inter-day and intra-day precision and accuracy are depicted in Table 1. The inter-day and intra-day accuracy (expressed as % relative error) and precision (expressed as %CV) for each concentration (LLOQ, LQC, MQC, and HQC) were within ±12.1% for the LLOQ and ±8.4% for all QC levels (for both plasma and Ringer’s solution samples).
Table 1.
Inter-day and intra-day precision and accuracy results for QC samples of ribociclib in mouse plasma (n=5) and Ringer’s solution (n=6)
| Matrix | Nominal (ng/ml) | Within-day
|
Between-day
|
||||
|---|---|---|---|---|---|---|---|
| Mean conc. | % CV | % Error | Mean conc. | % CV | % Error | ||
| Mouse Plasma | 7500 (HQC) | 7170.8 | 1.8 | −4.4 | 7306.0 | 4.4 | −2.6 |
| 650 (MQC) | 605.9 | 4.5 | −6.8 | 640.2 | 7.4 | −1.5 | |
| 187.5 (LQC) | 180.6 | 5.0 | −3.7 | 185.8 | 8.1 | −0.9 | |
| 62.5 (LLOQ) | 70.1 | 4.5 | 12.1 | 66.5 | 9.2 | 6.3 | |
| Ringer's solution | 75.0 (HQC) | 79.2 | 5.2 | 5.5 | 75.9 | 4.4 | 1.1 |
| 40.0 (MQC) | 42.6 | 6.6 | 6.6 | 41.8 | 4.9 | 4.5 | |
| 0.900 (LQC) | 0.854 | 8.3 | −5.1 | 0.869 | 8.4 | −3.5 | |
| 0.100 (LLOQ) | 0.105 | 4.9 | 5.0 | 0.111 | 7.9 | 11.1 | |
3.5 Lower limit of quantification and limit of detection
LLOQs were determined to be 62.5 ng/ml (S/N > 30) and 0.100 ng/ml (S/N > 13) for plasma and Ringer’s solution methods, respectively. LODs were 6.90 ng/ml (S/N >7) and 0.0500 ng/ml (S/N >3) for the mouse plasma and Ringer’s solution methods, respectively.
3.6 Selectivity, carry-over, and matrix effect
Selectivity was evaluated in both mouse plasma and Ringer’s solution and no endogenous peaks co-eluted with the components of interest in the different matrices. A typical chromatogram of blank mouse plasma and blank Ringer’s solution are depicted in Figs. 3 and 4. Additionally, chromatograms at assay LLOQ for mouse plasma and Ringer’s solution are also shown in Figs. 3 and 4. Chromatograms representing HQC and wash runs can be found in Supplementary Material (see Figs. S1, S2, S3, and S4). Carry-over was observed post injection of high concentration sample (i.e., ULQ & HQC) in Ringer’s solution assay development and ranged from 40–45% of the LLOQ for ribociclib and <0.3% for ISTD.
Carry-over seemingly was unavoidable in samples at higher concentrations. To account for this potential carry-over, we performed the following steps: (1) in samples with expected high concentrations (e.g., ULQ and HQC) four wash solutions were placed after the sample and before the next sample. We found that these wash injections reduced the carry-over to <15% (see Supplementary Material, Figs. S1 and S2); (2) Study samples with an expected a high concentration were diluted up to 3-fold to a concentration that lies towards the lower side of the curve and then injected into the LC-MS/MS system; (3) Two wash injections were placed approximately every fifth study sample injection. It is important to realize that the sample concentrations were expected to be low because these samples were diluted to get them within an expected range, so none of them were at the upper end of the calibration curve range. Carry-over value ranged from 0.5 – 15% of the LLOQ for the wash injections placed after study samples; (4) One set of QC (LQC, MQC & HQC) was placed at the end of the each run. The results of the QC samples at the beginning and end of the analytical runs consistently passed (e.g., within ±8% of the nominal concentration), and no significant carry-over was observed in any of those QC samples.
The matrix factor for the mouse plasma samples spiked with ribociclib at assay LQC (187.5 ng/ml) and HQC (7500 ng/ml), and ISTD (400 ng/ml) are shown in Table 2. This Table also summarizes the matrix factor for Ringer’s solution samples spiked with ribociclib at assay LQC (0.900 ng/ml) and HQC (75.0 ng/ml), and ISTD (5.00 ng/ml). As shown in Table 2, the chromatographic response of ribociclib and ISTD for Ringer’s solution was suppressed due to matrix effect, whereas the response of ribociclib and ISTD in plasma has minimal ion suppression. The presence of ionizable salts in the Ringer’s solution might be responsible for this ion suppression. Although ion suppression was observed, the ISTD normalized matrix factor for ribociclib remained constant over calibration curve range and Ringer’s solution method was both accurate and precise.
Table 2.
Matrix effect in different matrices
| Blank matrix ID | Ribociclib matrix factor * (Low QC) | ISTD matrix factor * | ISTD normalized matrix factor | Ribociclib matrix factor * (High QC) | ISTD matrix factor * | ISTD normalized matrix factor |
|---|---|---|---|---|---|---|
| Mouse Plasma | ||||||
| Source 1 | 0.97 ± 5.7 | 0.95 ± 2.4 | 1.02 | 1.02 ± 3.3 | 0.97 ± 7.5 | 1.05 |
| Mouse Plasma | ||||||
| Source 2 | 1.03 ± 4.6 | 0.93 ± 6.2 | 1.11 | 1.05 ± 1.9 | 0.99 ± 5.7 | 1.06 |
| Mouse Plasma | ||||||
| Source 3 | 1.02 ± 5.2 | 0.93 ± 9.6 | 1.10 | 1.09 ± 3.9 | 1.05 ± 2.2 | 1.04 |
| Ringer's Solution | 0.36 ± 21.2 | 0.39 ± 17.8 | 0.92 | 0.61 ± 21.8 | 0.60 ± 23.9 | 1.02 |
Results are presented as average ± CV% of triplicate
3.7 Stability
The results for the stability of ribociclib in mouse plasma and Ringer’s solution are depicted in Table 3. The result showed that ribociclib in mouse plasma was stable at 4ºC, room temperature, 37ºC for at least 24 h, and stable at −80ºC for at least 163 days. Ribociclib in Ringer’s solution was stable at 4°C, room temperature, 37°C for 7 h and stable at −80ºC for at least 40 days. The extract stability for mouse plasma and Ringer’s solution samples at 4 °C was confirmed to be at least 48 h (< 7% change) and 24 h (<1% change), respectively. The stock solutions were stable for at least 71 days (average percent difference was less than 4% between old stock and freshly made stock), when stored at −80 ºC.
Table 3.
Stability of ribociclib in mouse (CD-1) plasma and Ringer's solution
| Mouse Plasma
|
Ringer's Sol.
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Low QC % change
|
High QC % change
|
Low QC % change
|
High QC % change
|
||||||
| Bias | %CV | Bias | %CV | Bias | %CV | Bias %CV | |||
| 4°C | 4°C | ||||||||
| 4 h | −3.46 | 5.51 | 1.25 | 3.61 | 4 h | −6.82 | 1.29 | −3.29 | 1.63 |
| 7 h | 1.25 | 3.15 | 0.64 | 2.81 | 7 h | −4.93 | 0.80 | −0.46 | 1.74 |
| 24 h | 0.23 | 5.83 | 5.20 | 4.77 | 24 h | −18.14 | 2.03 | −4.17 | 2.46 |
| Room Temperature | Room Temperature | ||||||||
| 4 h | −3.58 | 3.17 | 0.39 | 2.07 | 4 h | −13.57 | 1.33 | −9.46 | 0.58 |
| 7 h | −0.07 | 2.73 | 2.35 | 11.40 | 7 h | −13.48 | 1.19 | −0.54 | 0.53 |
| 24 h | 6.82 | 4.77 | 5.99 | 8.17 | 24 h | −24.46 | 2.68 | −6.96 | 1.16 |
| 37°C | 37°C | ||||||||
| 4 h | −10.08 | 7.23 | −5.76 | 2.23 | 4 h | −14.90 | 1.13 | −5.55 | 2.17 |
| 7 h | 3.89 | 1.64 | −1.33 | 6.50 | 7 h | −11.40 | 4.80 | −6.04 | 2.66 |
| 24 h | −2.61 | 7.70 | −0.76 | 2.36 | 24 h | −16.16 | 1.46 | −10.49 | 2.24 |
| −80°C | −80°C | ||||||||
| 163 days | −7.76 | 9.50 | −5.56 | 2.87 | 40 days | 0.57 | 1.60 | −4.35 | 5.38 |
Results are presented as average and %CV of triplicate
3.8 Application of method to cerebral microdialysis study
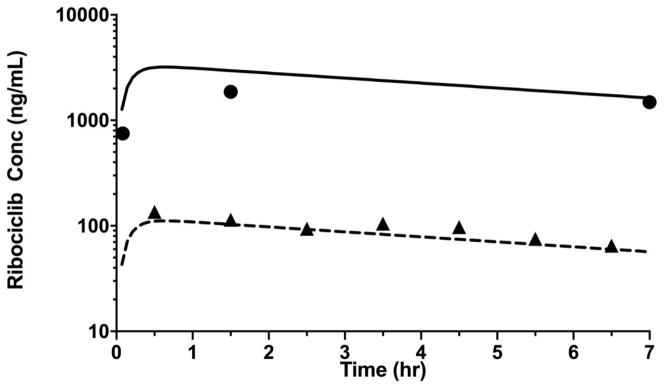
As shown in Fig. 5, we applied the methods developed here to measure ribociclib in mouse plasma and Ringer’s solution collected during a cerebral microdialysis study in a non-tumor bearing mouse. Using a noncompartmental pharmacokinetic analysis, we observed that ribociclib was rapidly absorbed after oral administration with a peak concentration observed within 1 h followed by a relatively slower elimination with a half-life of 6.4 h. Measurable ribociclib concentrations were observed in dialysate up to 7 h after oral administration. The brain ECF to plasma partition coefficient of ribociclib, a ratio of area under unbound ribociclib concentration-time curve for brain ECF to that for plasma was 0.17.
Figure 5.
Ribociclib concentration time profile in plasma and brain extracellular fluid (ECF) for the cerebral microdialysis experiment (closed circles represents observed ribociclib concentrations in plasma, and solid line represents model predicted concentration in plasma; closed triangle represents observed ribociclib concentrations in microdialysate, and dashed line represents model predicted concentration in microdialysate).
4. Conclusion
In conclusion, a simple and sensitive LC-MS/MS assay has been developed for the first time for quantitation of ribociclib in mouse plasma and Ringer’s solution. This method was linear, precise, and accurate within the given range. This assay requires only 20 μl volume of mouse plasma or 25 μl of Ringer’s solution for the quantitation of ribociclib. This assay can potentially be used to measure the concentration down to 0.100 ng/ml in Ringer’s solution and 62.5 ng/ml in mouse plasma with acceptable precision and accuracy. In addition, this method has been successfully applied for the determination of ribociclib concentration in preclinical samples.
Supplementary Material
Highlights.
A novel LC-MS/MS method for the quantitation of ribociclib in mouse plasma and Ringer’s solution was developed and validated.
Mouse plasma samples were extracted using solid phase extraction method, no extraction was required for the Ringer’s solution samples.
Method was successfully applied for the determination of ribociclib concentration in preclinical samples.
Acknowledgments
Research reported in the publication was supported by a Cancer Center Support (CORE) Grant CA 21765 and the American Lebanese Syrian Associated Charities (ALSAC).
Appendix A. Supplementary Material
Supplementary data associated with these assay methods can be found, in the online version.
Footnotes
Conflict of interest:
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro-oncology. 2012;14(Suppl 5):v1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson RA, Wright KD, Poppleton H, Mohankumar KM, Finkelstein D, Pounds SB, Rand V, Leary SE, White E, Eden C, Hogg T, Northcott P, Mack S, Neale G, Wang YD, Coyle B, Atkinson J, DeWire M, Kranenburg TA, Gillespie Y, Allen JC, Merchant T, Boop FA, Sanford RA, Gajjar A, Ellison DW, Taylor MD, Grundy RG, Gilbertson RJ. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature. 2010;466:632–636. doi: 10.1038/nature09173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohankumar KM, Currle DS, White E, Boulos N, Dapper J, Eden C, Nimmervoll B, Thiruvenkatam R, Connelly M, Kranenburg TA, Neale G, Olsen S, Wang YD, Finkelstein D, Wright K, Gupta K, Ellison DW, Thomas AO, Gilbertson RJ. An in vivo screen identifies ependymoma oncogenes and tumor-suppressor genes. Nature genetics. 2015;47:878–887. doi: 10.1038/ng.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson G, Parker M, Kranenburg TA, Lu C, Chen X, Ding L, Phoenix TN, Hedlund E, Wei L, Zhu X, Chalhoub N, Baker SJ, Huether R, Kriwacki R, Curley N, Thiruvenkatam R, Wang J, Wu G, Rusch M, Hong X, Becksfort J, Gupta P, Ma J, Easton J, Vadodaria B, Onar-Thomas A, Lin T, Li S, Pounds S, Paugh S, Zhao D, Kawauchi D, Roussel MF, Finkelstein D, Ellison DW, Lau CC, Bouffet E, Hassall T, Gururangan S, Cohn R, Fulton RS, Fulton LL, Dooling DJ, Ochoa K, Gajjar A, Mardis ER, Wilson RK, Downing JR, Zhang J, Gilbertson RJ. Novel mutations target distinct subgroups of medulloblastoma. Nature. 2012;488:43–48. doi: 10.1038/nature11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickson MA. Molecular pathways: CDK4 inhibitors for cancer therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20:3379–3383. doi: 10.1158/1078-0432.CCR-13-1551. [DOI] [PubMed] [Google Scholar]
- 6.Rader J, Russell MR, Hart LS, Nakazawa MS, Belcastro LT, Martinez D, Li Y, Carpenter EL, Attiyeh EF, Diskin SJ, Kim S, Parasuraman S, Caponigro G, Schnepp RW, Wood AC, Pawel B, Cole KA, Maris JM. Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:6173–6182. doi: 10.1158/1078-0432.CCR-13-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Infante JR, Cassier PA, Gerecitano JF, Witteveen PO, Chugh R, Ribrag V, Chakraborty A, Matano A, Dobson JR, Crystal AS, Parasuraman S, Shapiro GI. A Phase I Study of the Cyclin-Dependent Kinase 4/6 Inhibitor Ribociclib (LEE011) in Patients with Advanced Solid Tumors and Lymphomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22:5696–5705. doi: 10.1158/1078-0432.CCR-16-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghassabian S, Griffiths L, Smith MT. A novel fully validated LC-MS/MS method for quantification of pyridoxal-5'-phosphate concentrations in samples of human whole blood. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2015;1000:77–83. doi: 10.1016/j.jchromb.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Jacus MO, Throm SL, Turner DC, Patel YT, Freeman BB, 3rd, Morfouace M, Boulos N, Stewart CF. Deriving therapies for children with primary CNS tumors using pharmacokinetic modeling and simulation of cerebral microdialysis data. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2014;57:41–47. doi: 10.1016/j.ejps.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel YT, Jacus MO, Boulos N, Dapper JD, Davis AD, Vuppala PK, Freeman BB, 3rd, Mohankumar KM, Throm SL, Gilbertson RJ, Stewart CF. Preclinical examination of clofarabine in pediatric ependymoma: intratumoral concentrations insufficient to warrant further study. Cancer chemotherapy and pharmacology. 2015;75:897–906. doi: 10.1007/s00280-015-2713-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.