Abstract
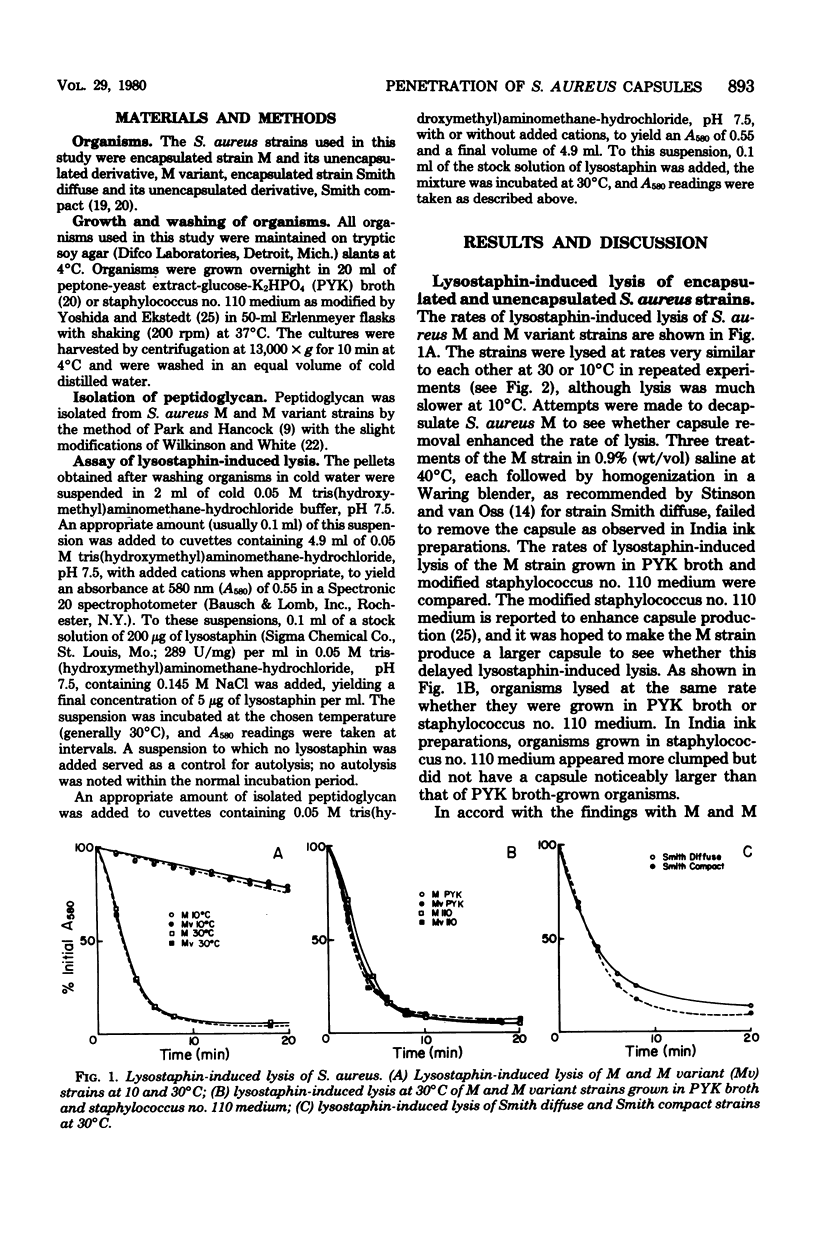
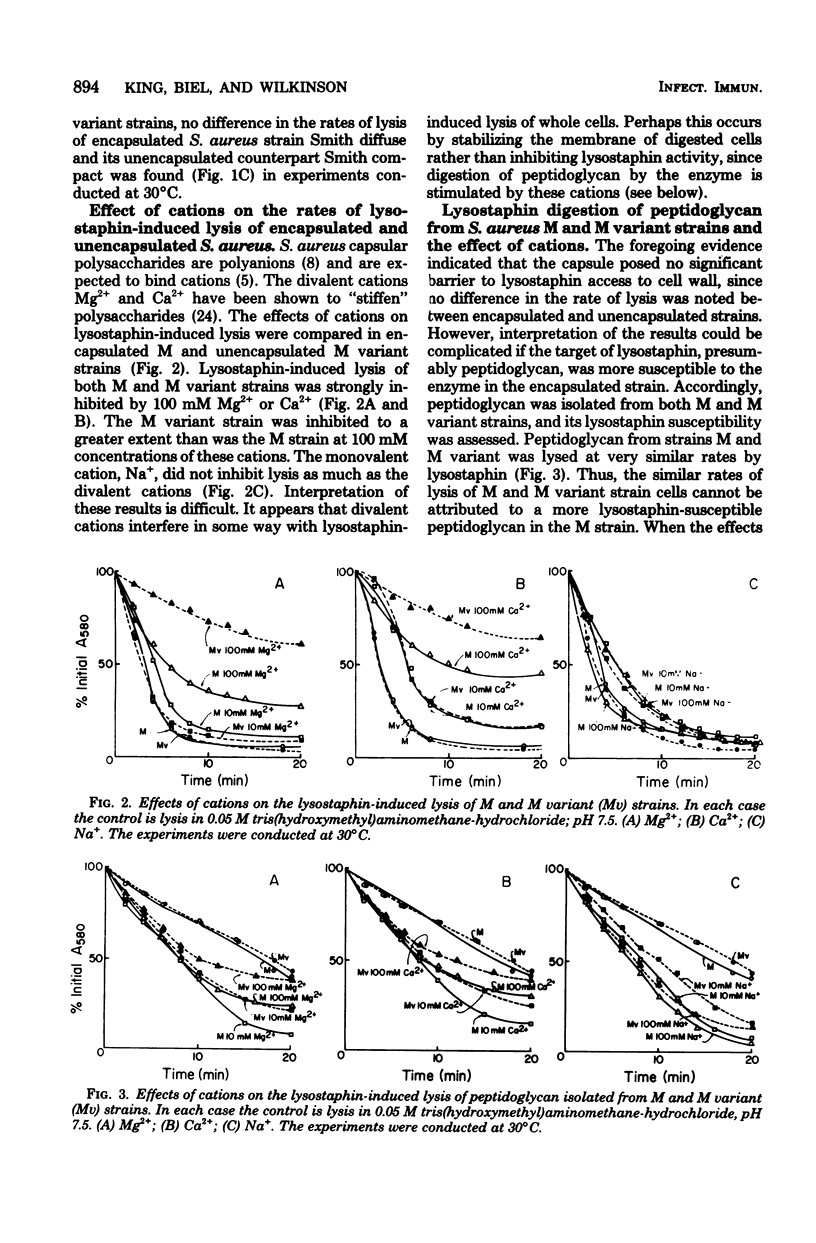
The ability of macromolecules to cross the capsular layer of encapsulated microorganisms and interact with their cell walls is important in considerations of the mechanisms of resistance to phagocytosis and of antigen masking in such strains. Lysostaphin was employed as a probe of the penetrability of the Staphylococcus aureus capsule. The rates of lysostaphin-induced lysis of encapsulated and unencapsulated S. aureus strains were compared. Encapsulated S. aureus strains M and Smith diffuse were lysed by lysostaphin at the same rate as their respective unencapsulated counterpart strains M variant and Smith compact. Growth of the M strain in a medium designed to enhance capsule production did not delay the onset or decrease the rate of lysis of the strain compared with organisms grown in normal medium. Cations did not selectively decrease the rate of lysis of the encapsulated strain, but inhibited the lysis of both the M and M variant strains. Peptidoglycan, the presumed lysostaphin target, isolated from both M and M variant strains was digested by lysostaphin at very similar rates. In contrast to whole cells, cations stimulated the rate of lysostaphin digestion of peptidoglycan. It is concluded that the fraction of lysostaphin active in cell lysis, believed to be a glycylglycine endopeptidase with a molecular weight of about 25,000, passes freely through the capsular layer to its target in the staphylococcal cell wall.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K., STEERE R. L. Restricted diffusion of macromolecules through agar-gel membranes. Biochim Biophys Acta. 1962 May 7;59:137–149. doi: 10.1016/0006-3002(62)90704-7. [DOI] [PubMed] [Google Scholar]
- Bernheimer H. P., Tiraby J. G. Inhibition of phage infection by pneumococcus capsule. Virology. 1976 Aug;73(1):308–309. doi: 10.1016/0042-6822(76)90085-4. [DOI] [PubMed] [Google Scholar]
- Glynn A. A., Howard C. J. The sensitivity to complement of strains of Escherichia coli related to their K antigens. Immunology. 1970 Mar;18(3):331–346. [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Influence of the Escherichia coli capsule on complement fixation and on phagocytosis and killing by human phagocytes. J Clin Invest. 1980 Jan;65(1):82–94. doi: 10.1172/JCI109663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau D. F., Hash J. H. Structural analysis of the surface polysaccharide of Staphylococcus aureus M. J Bacteriol. 1977 Jul;131(1):194–200. doi: 10.1128/jb.131.1.194-200.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- Robinson J. M., Hardman J. K., Sloan G. L. Relationship between lysostaphin endopeptidase production and cell wall composition in Staphylococcus staphylolyticus. J Bacteriol. 1979 Mar;137(3):1158–1164. doi: 10.1128/jb.137.3.1158-1164.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHINDLER C. A., SCHUHARDT V. T. LYSOSTAPHIN: A NEW BACTERIOLYTIC AGENT FOR THE STAPHYLOCOCCUS. Proc Natl Acad Sci U S A. 1964 Mar;51:414–421. doi: 10.1073/pnas.51.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D., Wheeler N., Laverdiere M., Blazevic D., Wilkinson B. J. A new type of penicillin resistance of Staphylococcus aureus. Lancet. 1977 Feb 26;1(8009):443–447. doi: 10.1016/s0140-6736(77)91941-9. [DOI] [PubMed] [Google Scholar]
- Scherrer R., Gerhardt P. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J Bacteriol. 1971 Sep;107(3):718–735. doi: 10.1128/jb.107.3.718-735.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Van Oss C. J. Immunoglobulins as aspecific opsonins. II. The influence of specific and aspecific immunoglobulins on the in vitro phagocytosis of noncapsulated, capsulated, and decapsulated bacteria by human neutrophils. J Reticuloendothel Soc. 1971 May;9(5):503–512. [PubMed] [Google Scholar]
- Sutherland I. W. Bacterial exopolysaccharides. Adv Microb Physiol. 1972;8:143–213. doi: 10.1016/s0065-2911(08)60190-3. [DOI] [PubMed] [Google Scholar]
- Trayer H. R., Buckley C. E., 3rd Molecular properties of lysostaphin, a bacteriolytic agent specific for Staphylococcus aureus. J Biol Chem. 1970 Sep 25;245(18):4842–4846. [PubMed] [Google Scholar]
- Verbrugh H. A., van Dijk W. C., van Erne M. E., Peters R., Peterson P. K., Verhoef J. Quantitation of the third component of human complement attached to the surface of opsonized bacteria: opsonin-deficient sera and phagocytosis-resistant strains. Infect Immun. 1979 Dec;26(3):808–814. doi: 10.1128/iai.26.3.808-814.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON J. F. The extracellualr polysaccharides of bacteria. Bacteriol Rev. 1958 Mar;22(1):46–73. doi: 10.1128/br.22.1.46-73.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Holmes K. M. Staphylococcus aureus cell surface: capsule as a barrier to bacteriophage adsorption. Infect Immun. 1979 Feb;23(2):549–552. doi: 10.1128/iai.23.2.549-552.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Peterson P. K., Quie P. G. Cryptic peptidoglycan and the antiphagocytic effect of the Staphylococcus aureus capsule: model for the antiphagocytic effect of bacterial cell surface polymers. Infect Immun. 1979 Feb;23(2):502–508. doi: 10.1128/iai.23.2.502-508.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Sisson S. P., Kim Y., Peterson P. K. Localization of the third component of complement on the cell wall of encapsulated Staphylococcus aureus M: implications for the mechanism of resistance to phagocytosis. Infect Immun. 1979 Dec;26(3):1159–1163. doi: 10.1128/iai.26.3.1159-1163.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., White P. J. The effect of antibiotics on synthesis of mucopeptide and teichoic acid by Pediococcus cerevisiae and by a substrain that requires methicillin for growth. J Gen Microbiol. 1973 Dec;79(2):195–204. doi: 10.1099/00221287-79-2-195. [DOI] [PubMed] [Google Scholar]
- Winter W. T., Arnott S. Hyaluronic acid: the role of divalent cations in conformation and packing. J Mol Biol. 1977 Dec 15;117(3):761–784. doi: 10.1016/0022-2836(77)90068-7. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Ekstedt R. D. Relation of mucoid growth of Staphylococcus aureus to clumping factor reaction, morphology in serum-soft agar, and virulence. J Bacteriol. 1968 Oct;96(4):902–908. doi: 10.1128/jb.96.4.902-908.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


