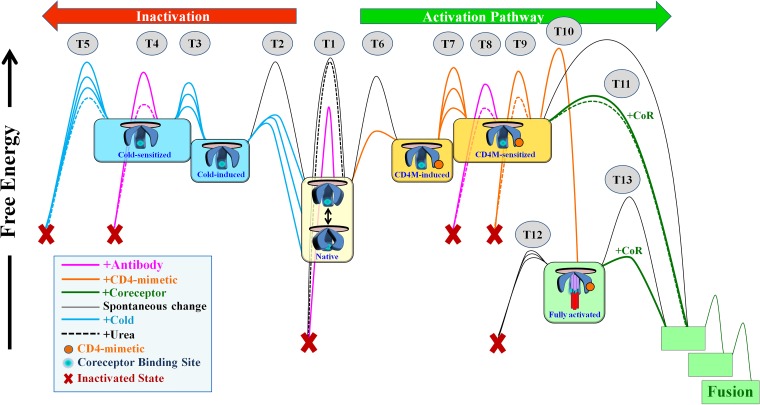
FIG 7.
Model of the energy landscape of HIV-1 Env. Native (cleaved and unliganded) Env trimers on the surfaces of HIV-1 particles potentially sample different conformations; at equilibrium, the sampling frequency of each conformation is determined by its free energy. The structural integrity of native and nonnative states is maintained by intramolecular interactions that create energy barriers and prevent changes to other forms, functional or nonfunctional. The stability of the states is thus determined by the strength of such interactions (i.e., the height of the activation barriers) and the presence of agents that can allow Envs to negotiate them. In diverse HIV-1 strains, different interactions prevent transitions (T) between states, accounting for the diverse patterns of responsiveness to Env-perturbing agents (represented by similarly colored barriers connecting states). Engagement of CD4Ms and coreceptor facilitates transitions to states along the activation pathway (directed to the right in the schematic). Env can also change to nonfunctional forms. Incubation at 0°C or engagement of CD4Ms can induce changes to states that expose the CoR-BS. Increased exposure to these agents facilitates transitions to sensitized forms, which are more readily neutralized by antibodies and eventually inactivate in a spontaneous manner. Such changes occur at isolate-specific rates and are reversible; removal of the CD4Ms or cold allows Env to return to the closed functional native state. The chaotropic agent urea perturbs the unstable forms induced by cold or CD4Ms and enhances their sensitivity to antibodies and their rate of spontaneous inactivation (dashed lines). CD4M-sensitized Envs are responsive to coreceptor (i.e., they can infect CD4− cells); at the range of concentrations applied in the study, transition to the sensitized form is reversible. Higher concentrations of CD4Ms induce irreversible changes to a state characterized by formation of the HR1 coiled coil, which is unstable and rapidly decays (within minutes) to a nonfunctional form unless coreceptor is engaged (33).