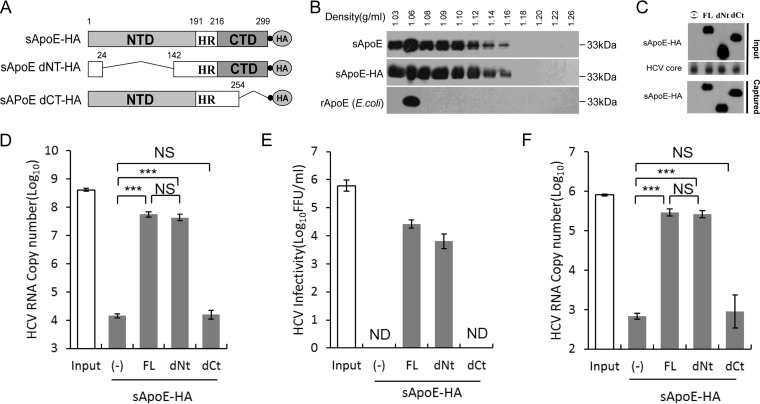
FIG 1.
Secreted ApoE interacts with extracellular HCV particles in a manner dependent on the C-terminal domain of ApoE. (A) Schematic representation of HA-tagged, full-length, N- or C-terminal domain deletion mutants of sApoE. (B) Isopycnic gradient centrifugation analysis showing the distribution of exogenously expressed sApoE or sApoE-HA in 293T cell culture medium as well as recombinant ApoE (rApoE) protein expressed by E. coli and run through a density gradient as a control. ApoE protein was detected by Western blotting with WUE-4 anti-ApoE antibody. (C) sApoE-HA and concentrated HCV core proteins in cell culture media were detected by Western blotting and are shown at the top. Proteins are specified on the left. The mixtures were separately captured by HA-specific immunoprecipitation, and the presence of sApoE-HA in the immunocomplexes was verified by Western blotting using HA antibody (bottom). (D) HCV genome copies in the immunocomplexes were quantified using qRT-PCR. (E) Determination of the infectivity of immunocaptured HCV particles. Equivalent amounts of HCVcc particles and full-length sApoE-HA or deletion mutants were mixed and subjected to HA affinity capture. Captured particles were eluted with an HA peptide. Infectivity titers in the input and the corresponding eluent were determined by FF-IHC. (F) PHH derived-HCV genomes in the immunocomplexes were pulled down using anti-HA agarose and quantified using qRT-PCR. The data in panels D to F represent the mean values and standard deviations from three independent experiments. **, P < 0.01; ***, P < 0.001 (unpaired Student's t test). NS, not significant; ND, not detected.