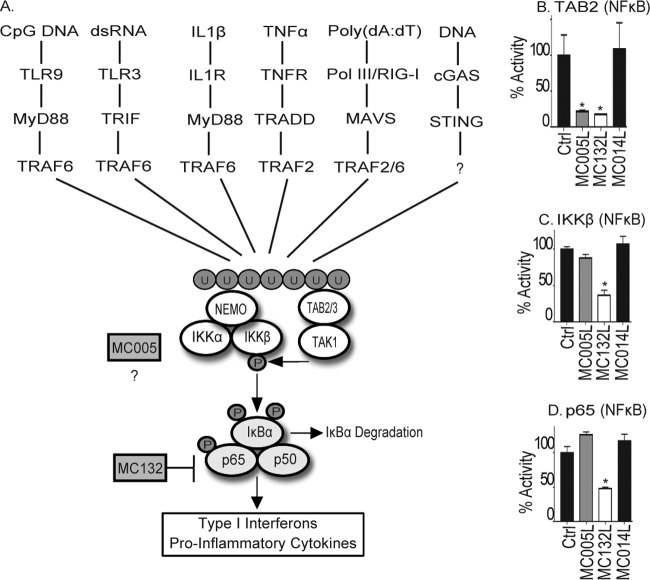
FIG 4.
MC005 inhibits PRR- and cytokine-stimulated NF-κB activation at a point proximal to the IKK complex. (A) Schematic showing multiple signal transduction pathways to NF-κB expected to be activated during a poxvirus infection, all of which are shown to be sensitive to MC005 inhibition (Fig. 1 to 3) and by MC132 targeting of p65. After the generation of ubiquitin chains, three key regulatory events occur that lead to canonical NF-κB activation. (i) The IKK complex (through NEMO) and the TAB/TAK complex (through the TABs) bind to ubiquitin chains. (ii) Ubiquitin binding of NEMO induces a conformational change that affects the entire IKK complex presenting the IKKs for autophosphorylation and phosphorylation by TAK1. (iii) Active phosphor-IKKβ dual phosphorylates both p65 and IκBα (triggering its degradation), leading to nuclear translocation of NF-κB and induction of antiviral and proinflammatory gene expression. (B to D) Comparison of MC005 with MC132 inhibition of signaling events surrounding IKK complex activation. HEK293T cells were seeded at 2 × 105/ml; transfected with 50 ng of the empty-vector (control [Ctrl]) or pCEP4 plasmid expressing the MCV ORFs indicated together with 10 ng of TAB2 (B), 10 ng of IKKβ (C), or 5 ng of p65 (D); harvested; and then assayed for NF-κB reporter gene activity 24 h later. Data are percentages of the stimulation activity in control cells and are the mean ± the standard deviation of triplicate samples from a representative experiment (n = 3). *, P < 0.001 compared to the control.