Abstract
Amino acids play critical roles in metabolism, cell function, body composition and immunity, but little data on plasma amino acid concentrations in HIV are available. We evaluated plasma amino acid concentrations and associations with CD4 counts and inflammatory biomarkers in HIV-infected youth. HIV-infected subjects with a high (≥500 cells/mm3) and low (<500 cells/mm3) current CD4+ T cell counts were compared to one another and to a matched healthy control group. Plasma concentrations of 19 amino acids were determined with an amino acid analyzer. Plasma levels of interleukin-6, tumor necrosis factor receptor-I, and soluble vascular cellular adhesion molecule-I were also measured. Seventy-nine HIV-infected subjects (40 and 39 with high and low CD4+ T cell counts, respectively) and 40 controls were included. There were no differences in amino acid concentrations between HIV-infected subjects with high or low CD4+ T cell counts. When combined, the HIV-infected group exhibited significantly lower median plasma concentrations compared to controls for total, essential, branched-chain and sulfur amino acids, as well as for 12 individual amino acids. Glutamate was the only amino acid that was higher in the HIV-infected group. There were no significant correlations between amino acid endpoints and inflammatory biomarkers for either HIV-infected group or controls. Plasma amino acid concentrations were lower in HIV-infected youth compared to healthy controls, regardless of immune status, while glutamate concentrations were elevated. These findings can inform future interventional studies designed to improve metabolic and clinical parameters influenced by amino acid nutriture.
Keywords: : pediatrics, amino acids, CD4 count, HIV
Introduction
Amino acids are the building blocks of proteins and play critical roles in metabolism, cell function, body composition, and immunity. Protein metabolism is commonly altered with severe illness, leading to changes in blood amino acid concentrations.1–3 These changes have been shown to have prognostic value in certain disease states. For example, decreased plasma citrulline and glutamine are independent predictors of mortality and associated with high inflammation in critically ill patients, and an increased level of the sulfur amino acid methionine is a prognostic factor for mortality in sepsis.1–3 Likewise, an increased plasma citrulline–arginine ratio is associated with adverse cardiovascular outcomes in children with chronic kidney failure.4 Amino acids are also important for T cell functions, serving as both a source of energy and biosynthetic precursors. It has been documented that glutamine uptake by T-lymphocytes increases upon their activation, and glutamine is well known to be a major fuel substrate for immune cells.5–7
Similar to other illnesses, HIV infection causes metabolic and net catabolic changes through processes, including increased metabolic rate, malabsorption, anorexia, and wasting syndrome.8 A small number of studies in the precombination antiretroviral therapy (cART) era showed that adults with HIV infection and AIDS have decreased plasma concentrations of amino acids.9–11 Notably, the few supplementation trials with glutamine, arginine, and sulfur-containing amino acids, respectively, showed improvements in various HIV-relevant endpoints, such as decreases in intestinal permeability in subjects with AIDS-associated diarrhea, increases in muscle mass and body weight in subjects with AIDS wasting, decreases in HIV-1 RNA levels, and increases in T cell counts.12–17
Little data exist, however, on plasma amino acid concentrations and HIV outcomes in the modern HIV era. Combination ART has transformed HIV into a chronic illness that allows people to live decades longer than before. Nevertheless, even with virologic suppression from cART, chronic HIV infection is associated with increased inflammation and immune activation, which contribute to ongoing immune dysfunction and comorbidities, including cardiovascular disease and HIV-associated neurocognitive disorder (HAND).18,19 Given the link between amino acids, inflammation, and immunity in other diseases, it is important to investigate these relationships in the HIV-infected population in the modern cART era. Such data could inform studies on specific amino acid supplementation and offer a therapeutic adjuvant to cART to improve immune function and attenuate HIV-related comorbidities.
Notably, amino acid concentrations have not been evaluated in HIV-infected children and young adults, a group whose metabolic needs are considerably different than in the adult population,20 yet still suffer from HIV-related comorbidities and immune dysfunction despite cART.21–24 Focusing on this younger population offers an opportunity for disease prevention and mitigation, as opposed to treatment of an established disease. Thus, the primary purpose of this observational study was to evaluate amino acid concentrations in HIV-infected children and young adults. Secondary objectives were to compare amino acid concentrations in HIV-infected subjects with a high versus low CD4+ T-cell count, compare amino acid concentrations to a group of matched healthy controls, and investigate the relationship between amino acid concentrations and levels of inflammatory and cardiovascular biomarkers.
Methods
Study design/population
HIV-infected subjects were selected a priori from a larger parent study investigating the vitamin D status of HIV-infected children and young adults and its relationship with markers of cardiovascular disease, inflammation, and immune restoration.25 This original study consisted of 200 HIV-infected children and young adults aged 1–25 years with documented HIV-1 infection, who received their medical care at the Ponce de Leon Youth HIV Clinic of the Grady Health System (Atlanta, GA). Exclusion criteria included current AIDS-defining clinical condition, inflammatory state besides HIV, or other chronic illness, such as noninfectious diarrhea. Patients with acute illnesses were eligible after complete resolution of symptoms for ≥1 month. Over 95% of approached patients consented to original study participation.
For this current analysis, the HIV-infected subjects whose current CD4+ T cell counts were in the lowest (5–234 cells/mm3) and highest (732–1,964 cells/mm3) quintile from the original study, respectively, were selected. Substitutions were made as necessary in the event that no stored plasma sample was available for a particular subject and so that, the two final HIV groups were matched by age, race, and sex. Eighty subjects in total were chosen: 40 with a high current CD4+ T cell count (≥500 cells/mm3) and 40 subjects with a low current CD4+ T cell count (<500 cells/mm3).
Forty healthy subjects were then selected from the original parent study of 50 controls, who matched the HIV-infected groups in age, race, and sex, and consisted of healthy relatives of HIV-infected patients seen at the clinic. Controls were 1–25 years old and self-reported to have no chronic disease or current/recent illness. For ages 12 and older, HIV-negative status was confirmed with OraQuick Advance Rapid HIV test (OraSure Technologies, Inc., Bethlehem, PA).
The study was reviewed and approved by the Institutional Review Boards of Emory University and Grady Health System. All participants and parents or legal guardians, if applicable, gave written consent to participate in the study. Children aged 6–10 years gave verbal assent, and those between 11 and 16 years gave written assent.
Clinical assessments
Demographic information was collected for both the HIV-infected and control groups by questionnaires. An extensive chart review was conducted for the HIV-infected subjects, including duration of HIV infection, detailed history of ART, past and current medical diagnoses, current medications, CD4+ T cell counts, and HIV-1 RNA level. Food intake over the previous 24 h was collected by conventional 24-hour recall by trained staff. Daily intake of macro- and micronutrient intake was determined by a registered dietitian in the Bionutrition Research Unit of the Atlanta Clinical and Translational Science Institute (ACTSI) using Nutrition Data System for Research (University of Minnesota, Minneapolis, MN), a dietary analysis software program for analysis of 24-h dietary recalls.
Laboratory assessments
All subjects fasted for eight or more hours before blood sampling. Plasma was obtained and stored at −80°C until analysis without prior thawing. All laboratory personnel were blinded to clinical information. Amino acid analysis was performed at the Emory University Genetics Laboratory using a Biochrom 30 Amino Acid Analyzer (Biochrom US, Holliston, MA). Nineteen amino acid concentrations in total were measured. Measured amino acids were considered one combined group (total amino acids), individual amino acids, and also grouped as measured essential amino acids (histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, and valine) that cannot be synthesized by humans, branched-chain amino acids (leucine, isoleucine, and valine) which are critical to muscle metabolism, and sulfur amino acids (cysteine and methionine) which are critical for redox metabolism, among other functions.26
Three key inflammatory and endothelial activation biomarkers were selected based on prior data showing them to be abnormally elevated in HIV and associated with heightened CVD risk and HIV-related comorbidities.27–30 These biomarkers were measured by the Luminex® System (Life Technologies, Grand Island, NY) and included soluble tumor necrosis factor receptor-1 (sTNFR-1) (Invitrogen, Carlsbad, CA), interleukin-6 (IL-6), and soluble vascular cellular adhesion molecule-1 (sVCAM-1) (Millipore, Billerica, MA).
Statistical methods
The HIV-infected group was initially analyzed based on current CD4+ T cell count (dichotomized as high, CD4+ T-cell ≥500 cells/mm3, and low, CD4+ T-cell <500 cells/mm3). Continuous measures are described by median/quartile (Q) 1, 3, or median/range, and nominal variables are described by frequency/percentage. Kruskal–Wallis test was used to compare the three groups (HIV-infected group with high CD4+ T-cell count, HIV-infected group with low CD4+ T-cell count, and healthy controls). Then, the HIV-infected group was combined and compared in toto to the healthy control group using nonparametric tests (Kruskal–Wallis for medians; chi-squared for categorical variables).
Spearman coefficients were used to assess correlations between selected amino acids, chosen based on potential clinical significance (total amino acids, essential amino acids, branched-chain amino acids, sulfur amino acids, arginine, glutamine, and citrulline), and inflammatory and cardiovascular biomarkers, BMI and CD4 count. Correlations were analyzed separately for the HIV-infected group (stratified by CD4+ T cell group and combined) and the healthy controls.
p values <.05 were considered significant in all cases except for correlations where a stricter p value of <.01 was used due to the large number of comparisons analyzed. All analyses were carried out using SAS, v.9.2 (The SAS Institute, Cary, NC).
Results
A total of 79 HIV-infected subjects (40 with a high CD4+ T cell count and 39 with a low CD4+ T cell count) and 40 healthy controls were included in the analysis; there was technical difficulty with one subject's sample from the low CD4+ T-cell count group, which led to the exclusion of this subject. All three groups were similar in age, sex, race, body mass index, and total intake of kilocalories/kg/day and protein/kg/day (Table 1). Median (range) of current CD4+ T cell counts in the low and high CD4+ T cell groups were 126 (5, 268) and 798 (580, 1,637) cells/mm3, respectively. Thirty-three (83%) subjects in the high CD4+ T cell group and 25 (64%) in the low CD4+ T cell group were on cART at the time of the study (p = .02). Seventy-five percent and 18% of subjects in the high CD4+ T cell and low CD4+ T cell group, respectively, had an undetectable HIV-1 RNA level (p < .01).
Table 1.
Demographics and Clinical Characteristics by Study Group
| HIV-infected subjects | ||||
|---|---|---|---|---|
| Median (range), or no. (%) | High CD4 (N = 40) | Low CD4 (N = 39) | Healthy controls (N = 40) | p |
| Age, years | 17 (8, 24) | 9 (10, 23) | 18 (9, 26) | .27 |
| Male sex, n (%) | 16 (40) | 22 (56) | 21 (52) | .31 |
| Black race, n (%) | 38 (95) | 35 (89) | 36 (90) | .53 |
| Body mass index, kg/m2 | 22 (15, 36) | 21 (15, 51) | 22 (17, 39) | .36 |
| Total kcal/kg/day intake | 30.1 (3.1, 107.7) | 33.5 (7.9, 65.9) | 29.7 (14.4, 56.2) | .99 |
| Total protein/kg/day intake, g | 1.3 (0.3, 3.7) | 1.1 (0.3, 2.6) | 1.0 (0.6, 2.3) | .79 |
| Current smoking | 4 (10) | 7 (18) | 4 (10) | .31 |
| Waist circumference, cm | 74 (50, 140) | 75 (59,116) | 76 (59, 99) | .96 |
| sVCAM-1, ng/ml | 1,226 (585, 2,796) | 1,424 (740, 3,459) | 1,094 (547, 1,826) | <.01 |
| Interleukin-6, ng/ml | 0 (0, 326) | 0 (0, 2) | 0 (0, 15) | .43 |
| sTNFR-1, pg/ml | 279 (40, 5,262) | 464 (108, 2,105) | 104 (29, 394) | <.01 |
| Current CD4 count, cells/mm3 | 798 (580, 1,637) | 126 (5, 268) | — | <.01 |
| CD4 nadir, cells/mm3 | 18 (3, 985) | 73 (2, 311) | — | <.01 |
| Perinatal transmission | 31 (78) | 23 (61) | — | .1 |
| Undetectable HIV-1 RNAa | 30 (75) | 7 (18) | — | <.01 |
| Currently on ART | 33 (83) | 25 (64) | — | .02 |
| CDC immunological category 3 | 16 (52) | 29 (85) | — | .01 |
Four subjects (two from each HIV group) had unknown detectable/undetectable HIV-1 RNA status at the time of the study.
ART, antiretroviral therapy; CDC, Centers for Disease Control; NNRTI, nonnucleoside reverse transcriptase inhibitor; sTNFR-I, soluble tumor necrosis factor receptor-I; sVCAM-1, soluble vascular cellular adhesion molecule-1.
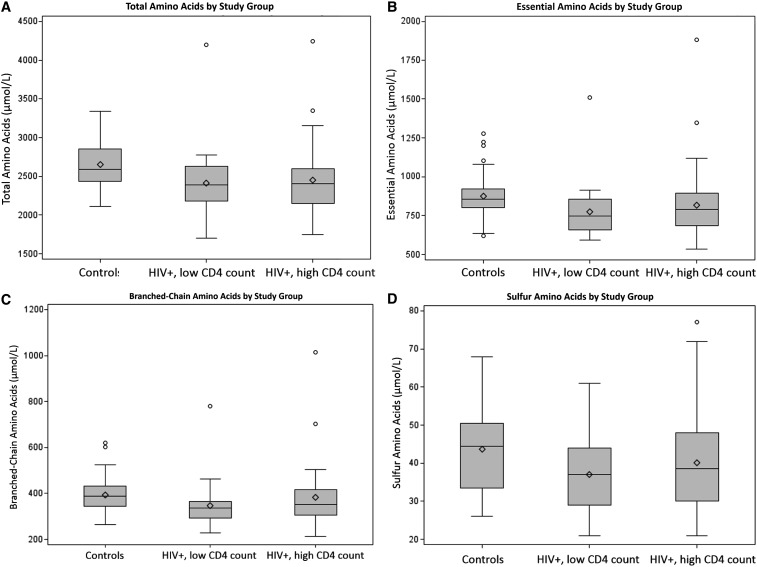
There were no significant differences for any of the individual amino acids or when grouped by clinical significance between the HIV-infected subjects with high CD4+ T cell counts versus low CD4+ T cell counts (Table 2). The two HIV groups were then combined and compared to the healthy controls. Compared to controls, HIV-infected subjects exhibited significantly lower median plasma concentrations for total amino acids, essential amino acids, branched-chain amino acids, and sulfur amino acids, despite similar total daily protein and kilocalorie intake per kg body weight per day (Table 2). Figure 1 shows the comparison of median plasma amino acid concentrations for the three groups: healthy controls, HIV-infected subjects with high CD4 count, and HIV-infected subjects with low CD4 count.
Table 2.
Plasma Amino Acid Concentrations for HIV-Infected Subjects Compared to Healthy Controls
| Median (Q1, Q3) μmol/L | Controls (N = 40) | High CD4 (N = 40) | Low CD4a(N = 39) | pb | All HIV+(N = 79) | pc |
|---|---|---|---|---|---|---|
| Total amino acids | 2,565 (2,431, 2,853) | 2,408 (2,146, 2,603) | 2,388 (2,181, 2,628) | .19 | 2,404 (1,698, 4,244) | .002 |
| Essential amino acids | 855 (800, 920) | 791 (683, 893) | 747 (657, 856) | .22 | 771 (535, 1,882) | <.001 |
| Branched-chain amino acids | 387 (338, 431) | 352 (306, 411) | 338 (294, 365) | .26 | 345 (213, 1,014) | .004 |
| Sulfur amino acids | 44 (33, 50) | 39 (30, 48) | 37 (29, 44) | .12 | 37 (21, 77) | .020 |
| Methionine | 25 (21, 27) | 20 (18, 23) | 20 (15, 22) | .03 | 20 (17, 23) | <.001 |
| Leucine | 117 (103, 130) | 109 (93, 127) | 97 (88, 115) | .24 | 103 (90, 116) | .001 |
| Citrulline | 32 (27, 34) | 29 (23, 33) | 27 (24, 30) | .02 | 27 (24, 33) | .002 |
| Valine | 215 (186, 237) | 193 (167, 224) | 185 (157, 208) | .20 | 186 (160, 217) | .004 |
| Proline | 168 (145, 185) | 147 (119, 174) | 139 (121, 176) | .33 | 143 (120, 175) | .005 |
| Aspartate | 5 (4, 6) | 4 (3, 6) | 4 (3, 6) | .04 | 4 (3, 6) | .009 |
| Glutamine | 529 (479, 583) | 459 (411, 538) | 498 (451, 554) | .07 | 481 (430, 552) | .011 |
| Lysine | 170 (148, 191) | 157 (132, 175) | 156 (138, 172) | .46 | 156 (136, 173) | .012 |
| Threonine | 135 (121, 173) | 129 (101, 152) | 131 (100, 160) | .09 | 129 (101, 155) | .013 |
| Tyrosine | 61 (53, 72) | 56 (46, 75) | 53 (42, 59) | .046 | 54 (45, 63) | .020 |
| Phenylalanine | 48 (43, 52) | 43 (39, 50) | 45 (40, 50) | .40 | 44 (40, 50) | .021 |
| Isoleucine | 58 (52, 66) | 54 (49, 67) | 52 (46, 60) | .28 | 53 (48, 63) | .045 |
| Serine | 114 (100, 130) | 108 (97, 115) | 103 (89, 125) | .18 | 105 (94, 121) | .057 |
| Alanine | 338 (306, 401) | 339 (280, 370) | 295 (242, 375) | .16 | 319 (255, 373) | .068 |
| Arginine | 51 (41, 65) | 44 (32, 57) | 50 (37, 66) | .37 | 45 (36, 62) | .091 |
| Histidine | 75 (71, 85) | 76 (72, 83) | 74 (66, 79) | .32 | 74 (66, 82) | .210 |
| Glycine | 259 (225, 308) | 252 (223, 278) | 265 (227, 288) | .69 | 258 (226, 285) | .482 |
| Ornithine | 76 (65, 102) | 77 (63, 103) | 72 (59, 89) | .33 | 72 (60, 94) | .555 |
| Cysteine | 19 (10, 26) | 17 (11, 24) | 16 (10, 23) | .90 | 16 (11, 24) | .589 |
| Glutamate | 60 (28, 78) | 71 (45, 91) | 70 (54, 81) | .08 | 71 (53, 88) | .022 |
Boldfaced p values indicate those <.05.
There were no p values <.05 for the comparison of HIV+ subjects with a high versus low CD4 count.
Comparison of the three groups (healthy controls, HIV+ with high CD4, and HIV+ with low CD4).
Comparison of all HIV+ subjects combined versus healthy controls).
FIG. 1.
Box and whisker plots for amino acid concentrations for HIV-infected subjects compared to controls. Box and whisker plots show concentrations for HIV-infected subjects grouped by high versus low CD4 cell count compared to controls for total amino acids (A), essential amino acids (B), branched-chain amino acids (C), and sulfur amino acids (D). In all cases, the HIV-infected subjects, regardless of CD4 count, had statistically lower concentrations than the controls.
When individual amino acids were examined, HIV-infected subjects had lower median plasma concentrations compared to the healthy controls for all of the amino acids except glutamate (Table 2). Plasma concentrations of 12 amino acids were statistically lower in the HIV-infected group: methionine, leucine, citrulline, valine, proline, aspartate, glutamine, lysine, threonine, tyrosine, phenylalanine, and isoleucine. Glutamate was the only amino acid with a higher plasma concentration in the HIV-infected group compared to the controls. Table 3 shows the age-related normal values for each plasma amino acid and the percentage of subjects by study group who were below the normal range for their age. The HIV-infected group had a higher percentage of subjects below the normal range for all amino acids except glycine.
Table 3.
Percentage of Subjects by Study Group Below the Age-Related Normal Plasma Amino Acid Values
| % of subjects below the normal range | Normal range by age, μmol/L | |||||
|---|---|---|---|---|---|---|
| Amino acid | High CD4 | Low CD4 | All HIV+ | Controls | 2–18 years | >18 years |
| Phenylalanine | 30 | 39 | 34 | 18 | 26–91 | 49–76 |
| Methionine | 18 | 36 | 27 | 10 | 7–47 | 21–41 |
| Alanine | 15 | 23 | 19 | 5 | 152–547 | 281–565 |
| Lysine | 10 | 23 | 17 | 5 | 48–284 | 148–235 |
| Valine | 7.5 | 15 | 12 | 2.5 | 74–321 | 164–275 |
| Tyrosine | 7.5 | 15 | 11 | 2.5 | 24–115 | 41–78 |
| Arginine | 5 | 18 | 11 | 2.5 | 10–140 | 35–154 |
| Leucine | 5 | 15 | 10 | 2.5 | 49–216 | 88–158 |
| Ornithine | 7.5 | 10 | 8.9 | 5 | 10–163 | 59–155 |
| Cysteine | 5 | 10 | 7.6 | 7.5 | 5–45 | 8–83 |
| Proline | 7.5 | 7.7 | 7.6 | 0 | 59–369 | 103–284 |
| Glycine | 5 | 7.7 | 6.3 | 10 | 127–341 | 196–400 |
| Threonine | 5 | 5 | 5 | 0 | 35–226 | 85–186 |
| Isoleucine | 5 | 5.1 | 3.8 | 2.5 | 22–107 | 40–83 |
| Histidine | 0 | 7.7 | 3.8 | 0 | 41–125 | 52–128 |
| Citrulline | 0 | 2.6 | 1.3 | 0 | 1–46 | 11–50 |
| Taurine | 2.5 | 0 | 1.2 | 0 | 10–170 | 40–150 |
| Serine | 2.5 | 0 | 1.2 | 0 | 69–187 | 76–161 |
| Glutamate | 0 | 0 | 0 | 0 | 5–150 | 10–131 |
| Glutamine | 0 | 0 | 0 | 0 | 254–823 | 205–756 |
| Aspartate | 0 | 0 | 0 | 0 | 1–24 | 0–13 |
Next, correlations were examined between the plasma amino acid concentrations (total amino acids, essential amino acids, branched-chain amino acids, sulfur amino acids, arginine, glutamine, and citrulline) and inflammatory and cardiovascular biomarkers (IL-6, sTNFR-1, and sVCAM-1) for both the HIV-infected groups and the control group. Correlations were also determined between CD4 count and the amino acids for the HIV-infected group combined, as well as for the high and low CD4 groups separately. Finally, correlations were evaluated between the amino acids and BMI for all HIV-infected subjects and controls combined, for the healthy controls separately, and then for the HIV-infected subjects as one group and separated by CD4 count group. There were no significant correlations found at the selected p-value of <.01.
After the initial results were known, the HIV-infected group was then divided based on those with and without an undetectable HIV-1 RNA level. There was no difference between these two HIV groups in any of the amino acid endpoints examined (total amino acids, essential amino acids, branched-chain amino acids, sulfur amino acids, arginine, citrulline, glutamate, and glutamine). There were also no significant correlations between these amino acid concentrations and any of the inflammatory or CVD biomarkers when the HIV-infected group was considered by HIV-1 RNA status. The correlations between plasma glutamate concentrations and the biomarkers were also considered with the HIV-infected group combined, but were not significant.
Discussion
This study is the largest, to date, to determine plasma amino acid status in HIV-infected youth, and the first to compare these values to healthy controls and explore relationships among amino acid concentrations and inflammatory biomarkers. Among the HIV-infected population, amino acid plasma concentration did not differ by CD4 categories or HIV-1 RNA status (undetectable vs. detectable). However, overall, the HIV-infected youth had lower plasma concentrations compared to healthy controls for total, essential, branched-chain, and sulfur amino acids, as well as all of the individual amino acids except glutamate.
Although previous studies in HIV-infected adults have reported similar findings of decreased plasma amino acid concentrations, the underlying reasons for this finding have not been fully elucidated. The lower concentrations we report in HIV-infected youth versus healthy uninfected controls could potentially be explained by differences in intestinal absorption, amino acid utilization, and/or amino acid requirements; however, further studies are required to delineate these possibilities. In one small pre-cART study investigating protein metabolism in six HIV-infected children, there was an increased protein catabolism compared to the controls, but no difference in protein synthesis. The authors suggested that this difference in protein catabolism may have been due to lower energy intakes in the HIV-infected group.20 In this study, however, there were no significant differences in dietary caloric or protein intake between the two groups, implying another mechanism for the differences between groups we observed for the plasma amino acid concentrations.
Another small study of eight HIV-infected children also showed that protein catabolism was higher in the HIV-infected group, despite no difference in dietary protein intake between the two groups. Protein catabolism decreased after 6 weeks of protease inhibitor therapy, but did not decrease to the range found in the control children.31 This study and these findings suggest that HIV-infected children may have higher rates of protein turnover even with antiretroviral therapy and virologic control and may need greater dietary energy and/or high-quality protein intakes to improve their protein balance. This may also have positive effects on immune function, given the important role of protein nutriture on immune functions,32,33 and this would be of interest to investigate in future studies in populations with HIV infection such as ours.
It is also worth noting that, although speculative, the decreased concentrations of plasma amino acids may be due to altered intestinal absorption. In chronic HIV infection, mucosal production of inflammatory cytokines is associated with increased epithelial permeability, epithelial apoptosis, and alterations of epithelial tight junctions,34 which could certainly impair amino acid absorption.
It is difficult to determine the clinical significance of these findings since our study was not designed to answer that question and there are no data from other populations that can be directly compared to ours. However, a number of studies in HIV with similar general findings suggest that our data are potentially clinically meaningful and should be further explored. For example, in a relatively recent study, plasma citrulline levels, which are specifically synthesized by small-bowel enterocytes (using the amino acid glutamine as a precursor), were a reliable biomarker of enteropathy in HIV patients and deemed to be an objective tool for assessing the need for parenteral nutrition in this population.35 The compromised intestinal mucosal barrier associated with chronic HIV infection also leads to microbial translocation. The microbe themselves and their products (e.g., lipopolysaccharide and flagellin) circulate in the plasma, upregulate various receptors (e.g., toll-like receptors 4 and 5, respectively), and may thus contribute to immune activation.36 Increased immune activation independently predicts mortality in HIV-infected adults.37–41 Amino acid supplementation with glutamine has been shown to improve intestinal permeability in AIDS patients.12,42 Thus, given these data, investigating the effects of amino acid supplementation not only on plasma concentrations of amino acids but also on microbial translocation and immune activation may be worthwhile.
Notably, we found that plasma glutamate concentrations were higher in the HIV-infected group compared to the healthy controls, which is consistent with previous studies in HIV-infected adults.43,44 Altered glutamate metabolism in the brain has been linked with HAND, neurological disorders associated with HIV infection that encompass a wide spectrum of disease severity from AIDS dementia complex to mild neurocognitive disorder, in the adult HIV-infected population.45 HAND is characterized by cognitive impairments that according to recent studies affect 15%–50% of people infected with HIV despite virologic suppression and immune reconstitution.46,47 The etiology of HAND is still poorly understood, but it is related to neuronal damage caused by heightened inflammation and immune activation associated with chronic HIV. One hypothesis is that activated macrophages contribute to neuronal injury by producing excess extracellular glutamate. This coupled with a decrease in astrocytic reuptake of glutamate causes a net loss of intracellular glutamate into the extracellular space.48,49
One pre-cART study did not find an association between increased plasma glutamate levels and HIV-associated dementia,43 suggesting that cerebral spinal fluid glutamate concentrations may be increased by other mechanisms besides an increased transport of the amino acid from the plasma across an altered blood–brain barrier. Thus, studies which relate serial glutamate levels in plasma, coupled with dietary assessment as performed in this study, to the development of HAND in HIV-infected subjects would be of interest to delineate the potential biomarker and/or pathophysiologic role of plasma glutamate in this complication.
Increased plasma glutamate concentrations appear to also have clinical implications outside of the central nervous system. In vitro data show that elevated extracellular glutamate concentrations inhibit lymphocyte and macrophage function, and a fourfold increase in extracellular glutamate concentration causes a 30%–40% inhibition of DNA synthesis. Importantly, the inhibitory effect of glutamate was largely compensated for by the addition of cysteine.44
Likewise, several studies have shown that HIV-infected adults have a deficiency of antioxidant nutrients, including glutathione (a major player in antioxidant defenses and immune responses), which may contribute to disease progression.50 Oral supplementation with cysteine, the rate-limiting step in the synthesis of glutathione, has shown to increase concentrations of glutathione in the plasma, muscle, and bronchoalveolar lavage fluid in this population. Similarly, oral glutamine supplementation improves plasma glutathione concentrations and significantly increases lean body mass.51 In another study of HIV-infected older adults, glutathione deficiency secondary to impaired synthesis was restored with cysteine plus glycine supplementation. Glutathione improvement was accompanied by marked improvements in mitochondrial fuel oxidation, insulin sensitivity, body composition, anthropometry, muscle strength, and dyslipidemia,52 all metabolic disorders also known to affect HIV-infected children and young adults.53,54
In this study, we also investigated associations between individual amino acid concentrations and specific inflammatory and cardiovascular markers. Previous studies evaluating children with other disease states have shown an association between the plasma concentrations of arginine and citrulline and markers of inflammation.55 We did not detect any significant correlation with the amino acids and inflammatory biomarkers measured in these HIV-infected children and young adults. It may be that there is a more robust link between amino acid levels in blood and immune activation rather than inflammation in the HIV-infected population, as evidenced by a recent study showing the importance of the monocyte activation marker, soluble CD14 in HAND.56 That study showed that the combined concentrations of glutamate and glutamine were significantly associated with soluble levels of CD14 in both the plasma and cerebrospinal fluid.
There are several important limitations to this study, including a relatively small sample size, multiple comparisons, and a cross-sectional design, which limit the conclusions one can draw from these data, including cause and effect relationships. In addition, the subjects spanned a wide age range and comprised a heterogeneous HIV-infected population, including some subjects who were not on cART and/or had a detectable HIV-1 RNA level at the time of the study, limiting generalizability. We tried to adjust for this, however, by reanalyzing the HIV-infected group by HIV-1 RNA status, which did not change the study results. Also, for technical reasons, this study did not include tryptophan measurements, which may play important roles in HIV-related comorbidities.57–59 Likewise, our study selected only a few inflammatory biomarkers to examine, which may not be the most important ones associated with amino acids in HIV infection. Finally, although we were able to show that HIV-infected children and young adults have decreased plasma concentrations of amino acids compared to healthy controls, our study was not designed to determine the underlying cause of this finding or whether there is clinical relevance.
Nevertheless, these exploratory data offer insight into amino acid metabolism in this understudied population. To our knowledge, this is the first study to measure amino acid concentrations in HIV-infected children and young adults. These results are novel and suggest that additional prospective studies, coupled with detailed dietary intake data, would be valuable to assess the clinical significance of these findings and determine if supplementation trials produce positive results. Studying these elements in HIV-infected youth is an innovative approach to identify potential nutrition-related prevention strategies designed to mitigate HIV-related comorbidities and HIV disease progression.
Acknowledgments
This work was supported by research grants from GlaxoSmithKline, Emory-Egleston Children's Research Center, and Emory's Center for AIDS Research (P30 AI050409); the National Institute of Child Health and Development at the National Institutes of Health (K23 HD069199 to ARE and R01 HD070490 to GAM), Atlanta Clinical and Translational Science Institute, National Center for Advancing Translational Sciences of the National Institutes of Health (UL1 TR000454), and the National Institute of Diabetes, Digestive and Kidney Disease at the National Institutes of Health (K24 DK096574 to TRZ). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data, in part, were presented previously at the 15th International Workshop on Comorbidities and Adverse Drug Reactions in HIV, Brussels, Belgium, October 2013. Abstract 37.
Author Disclosure Statement
A.R.E. has received research funding from Bristol-Myers Squibb, Cubist Pharmaceuticals, and GlaxoSmithKline, and has served as an advisor and speaker for Gilead. G.A.M. serves as a consultant, speaker, and has received research funding from Bristol-Myers Squibb, ViiV/GlaxoSmithKline, Gilead, ICON, and Merck. All other authors declare no conflicts of interest.
References
- 1.Piton G, Manzon C, Monnet E, et al. : Plasma citrulline kinetics and prognostic value in critically ill patients. Intensive Care Med 2010;36:702–706 [DOI] [PubMed] [Google Scholar]
- 2.Oudemans-van Straaten H, Bosman R, Treskes M, van der Spoel H, Zandstra D: Plasma glutamine depletion and patient outcome in acute ICU admissions. Intensive Care Med 2001;27:84–90 [DOI] [PubMed] [Google Scholar]
- 3.Hirose T, Shimizu K, Ogura H, et al. : Altered balance of the aminogram in patients with sepsis—The relation to mortality. Clin Nutr 2014;33:179–182 [DOI] [PubMed] [Google Scholar]
- 4.Lin Y-J, Hsu C-N, Lo M-H, Huang C-F, Chien S-J, Tain Y-L: High citrulline-to-ariginine ratio associated with blood pressure abnormalities in children with early chronic kidney disease. Circ J 2013;77:181–187 [DOI] [PubMed] [Google Scholar]
- 5.Carr E, Kelman A, Wu G, et al. : Glutamate uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J Immunol 2010;185:1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newsholme P: Why is L-glutamate metabolism important to cells of the immune system in health, postinjury, surgery or infection? J Nutr 2001;131:2515S–2522S [DOI] [PubMed] [Google Scholar]
- 7.Yaqoob P, Calder PC: Glutamine requirement of proliferating T lymphocytes. Nutrition 1997;13:646–651 [DOI] [PubMed] [Google Scholar]
- 8.Grunfeld C, Feingold KR: Metabolic disturbances and wasting in the acquired immunodeficiency syndrome. N Engl J Med 1992;327:329–336 [DOI] [PubMed] [Google Scholar]
- 9.Stein T, Nutinsky C, Condoluci D, Schluter M, Leskiw M: Protein and energy substrate metabolism in AIDS patients. Metabolism 1990;39:876–881 [DOI] [PubMed] [Google Scholar]
- 10.Hortin GL, Landt M, Powderly WG: Changes in plasma amino acid concentrations in response to HIV-1 infection. Clin Chem 1994;40:785–789 [PubMed] [Google Scholar]
- 11.Droge W, Eck H, Gmunder H, Mihm S: Dysregulation of plasma amino acid levels in HIV-infection and cancer and its relevance for the immune system. Amino Acids 1991;1:193–198 [DOI] [PubMed] [Google Scholar]
- 12.Noyer CM, Simon D, Borczuk A, Brandt LJ, Lee MJ, Nehra V: A double-blind placebo-controlled pilot study of glutamine therapy for abnormal intestinal permeability in patients with AIDS. Am J Gastroenterol 1998;93:972–975 [DOI] [PubMed] [Google Scholar]
- 13.New supplement treats AIDS wasting successfully. AIDS Alert 1999;14:41–43 [PubMed] [Google Scholar]
- 14.Shabert JK, Winslow C, Lacey JM, Wilmore DW: Glutamine-antioxidant supplementation increases body cell mass in AIDS patients with weight loss: A randomized, double-blind controlled trial. Nutrition 1999;15:860–864 [DOI] [PubMed] [Google Scholar]
- 15.Breitkreutz R, Pittack N, Nebe CT, et al. : Improvement of immune functions in HIV infection by sulfur supplementation: Two randomized trials. J Mol Med 2000;78:55–62 [DOI] [PubMed] [Google Scholar]
- 16.Suttmann U, Ockenga J, Schneider H, et al. : Weight gain and increased concentrations of receptor proteins for tumor necrosis factor after patients with symptomatic HIV infection received fortified nutrition support. J Am Diet Assoc 1996;96:565–569 [DOI] [PubMed] [Google Scholar]
- 17.Rohde T, Ullum H, Rasmussen JP, Kristensen JH, Newsholme E, Pedersen BK: Effects of glutamine on the immune system: Influence of muscular exercise and HIV infection. J Appl Physiol 1995;79:146–150 [DOI] [PubMed] [Google Scholar]
- 18.Triant VA, Lee H, Hadigan C, Grinspoon SK: Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007;92:2506–2512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clifford DB: HIV-associated neurocognitive disease continues in the antiretroviral era. Top HIV Med 2008;16:94–98 [PubMed] [Google Scholar]
- 20.Jahoor F, Abramson S, Heird WC: The protein metabolic response to HIV infection in young children. Am J Clin Nutr 2003;78:182–189 [DOI] [PubMed] [Google Scholar]
- 21.Rosenblatt HM, Stanley KE, Song LY, et al. : Immunological response to highly active antiretroviral therapy in children with clinically stable HIV-1 infection. J Infect Dis 2005;192:445–455 [DOI] [PubMed] [Google Scholar]
- 22.Rainwater-Lovett K, Nkamba H, Mubiana-Mbewe M, Moore CB, Margolick J, Moss WJ: Changes in cellular immune activation and memory T-cell subsets in HIV-infected Zambian children receiving HAART. J Acquir Immune Defic Syndr 2014;67:455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapetanovic S, Leister E, Nichols S, et al. : Relationships between markers of vascular dysfunction and neurodevelopmental outcomes in perinatally HIV-infected youth. AIDS 2010;24:1481–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross AC, Storer N, O'Riordan MA, Dogra V, McComsey GA: Longitudinal changes in carotid intima-media thickness and cardiovascular risk factors in human immunodeficiency virus-infected children and young adults compared with healthy controls. Pediatr Infect Dis J 2010;29:634–638 [DOI] [PubMed] [Google Scholar]
- 25.Eckard A, Juda S, Ziegler T, et al. : Risk factors for vitamin D deficiency and relationship with cardiac biomarkers, inflammation and immune restoration in HIV-infected youth. Antivir Ther 2012;17:1069–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews D: Protein and amino acids. In: Modern Nutrition in Health and Disease (Ross AC, ed.) 11th edition/ed. Wolters Kluwer Health/Lippincott Williams & Wilkins, Philadelphia, 2014, pp. 3–35 [Google Scholar]
- 27.Pai JK, Pischon T, Ma J, et al. : Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med 2004;351:2599–2610 [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR: Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 2002;347:1557–1565 [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E: Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation 2000;101:2149–2153 [DOI] [PubMed] [Google Scholar]
- 30.Hwang SJ, Ballantyne CM, Sharrett AR, et al. : Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 1997;96:4219–4225 [DOI] [PubMed] [Google Scholar]
- 31.Hardin DS, Ellis KJ, Rice J, Doyle ME: Protease inhibitor therapy improves protein catabolism in prepubertal children with HIV infection. J Pediatr Endocrinol Metab 2004;17:321–325 [DOI] [PubMed] [Google Scholar]
- 32.Chandra RK: Nutrition and the immune system: An introduction. Am J Clin Nutr 1997;66:460S–463S [DOI] [PubMed] [Google Scholar]
- 33.Sobrado J, Maiz A, Kawamura I, Moldawer LL, Bistrian BR, Blackburn GL: Effect of dietary protein depletion on nonspecific immune responses and survival in the guinea pig. Am J Clin Nutr 1983;37:795–801 [DOI] [PubMed] [Google Scholar]
- 34.Epple HJ, Zeitz M: HIV infection and the intestinal mucosal barrier. Ann N Y Acad Sci 2012;1258:19–24 [DOI] [PubMed] [Google Scholar]
- 35.Crenn P, De Truchis P, Neveux N, Galperine T, Cynober L, Melchior JC: Plasma citrulline is a biomarker of enterocyte mass and an indicator of parenteral nutrition in HIV-infected patients. Am J Clin Nutr 2009;90:587–594 [DOI] [PubMed] [Google Scholar]
- 36.Nasi M, Pinti M, Mussini C, Cossarizza A: Persistent inflammation in HIV infection: Established concepts, new perspectives. Immunol Lett 2014;161:184–188 [DOI] [PubMed] [Google Scholar]
- 37.Brenchley JM, Price DA, Schacker TW, et al. : Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 2006;12:1365–1371 [DOI] [PubMed] [Google Scholar]
- 38.Jiang W, Lederman MM, Hunt P, et al. : Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis 2009;199:1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sandler NG, Wand H, Roque A, et al. : Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011;203:780–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Justice AC, Freiberg MS, Tracy R, et al. : Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis 2012;54:984–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunt PW, Sinclair E, Rodriguez B, et al. : Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014;210:1228–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leite RD, Lima NL, Leite CA, Farhat CK, Guerrant RL, Lima AA: Improvement of intestinal permeability with alanyl-glutamine in HIV patients: A randomized, double blinded, placebo-controlled clinical trial. Arq Gastroenterol 2013;50:56–63 [DOI] [PubMed] [Google Scholar]
- 43.Ferrarese C, Aliprandi A, Tremolizzo L, et al. : Increased glutamate in CSF and plasma of patients with HIV dementia. Neurology 2001;57:671–675 [DOI] [PubMed] [Google Scholar]
- 44.Eck HP, Frey H, Droge W: Elevated plasma glutamate concentrations in HIV-1-infected patients may contribute to loss of macrophage and lymphocyte functions. Int Immunol 1989;1:367–372 [DOI] [PubMed] [Google Scholar]
- 45.Vazquez-Santiago FJ, Noel RJ, Jr., Porter JT, Rivera-Amill V: Glutamate metabolism and HIV-associated neurocognitive disorders. J Neurovirol 2014;20:315–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cysique LA, Maruff P, Brew BJ: Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: A combined study of two cohorts. J Neurovirol 2004;10:350–357 [DOI] [PubMed] [Google Scholar]
- 47.Simioni S, Cavassini M, Annoni JM, et al. : Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS 2010;24:1243–1250 [DOI] [PubMed] [Google Scholar]
- 48.Zhao L, Huang Y, Tian C, et al. : Interferon-alpha regulates glutaminase 1 promoter through STAT1 phosphorylation: Relevance to HIV-1 associated neurocognitive disorders. PLoS One 2012;7:e32995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L: Lower brain glutamate is associated with cognitive deficits in HIV patients: A new mechanism for HIV-associated neurocognitive disorder. J Magn Reson Imaging 2010;32:1045–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bogden JD, Kemp FW, Han S, et al. : Status of selected nutrients and progression of human immunodeficiency virus type 1 infection. Am J Clin Nutr 2000;72:809–815 [DOI] [PubMed] [Google Scholar]
- 51.Borges-Santos MD, Moreto F, Pereira PC, Ming-Yu Y, Burini RC: Plasma glutathione of HIV(+) patients responded positively and differently to dietary supplementation with cysteine or glutamine. Nutrition 2012;28:753–756 [DOI] [PubMed] [Google Scholar]
- 52.Nguyen D, Hsu JW, Jahoor F, Sekhar RV: Effect of increasing glutathione with cysteine and glycine supplementation on mitochondrial fuel oxidation, insulin sensitivity, and body composition in older HIV-infected patients. J Clin Endocrinol Metab 2014;99:169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eckard AR, Fowler SL, Haston JC, Dixon TC: Complications of treatment in youth with HIV. Curr HIV/AIDS Rep 2016;13:226–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barlow-Mosha L, Eckard AR, McComsey GA, Musoke PM: Metabolic complications and treatment of perinatally HIV-infected children and adolescents. J Int AIDS Soc 2013;16:18600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Waardenburg DA, de Betue CT, Luiking YC, Engel M, Deutz NE: Plasma arginine and citrulline concentrations in critically ill children: Strong relation with inflammation. Am J Clin Nutr 2007;86:1438–1444 [DOI] [PubMed] [Google Scholar]
- 56.Anderson AM, Harezlak J, Bharti A, et al. : Plasma and cerebrospinal fluid biomarkers predict cerebral injury in HIV-infected individuals on stable combination antiretroviral therapy. J Acquir Immune Defic Syndr 2015;69:29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jenabian MA, El-Far M, Vyboh K, et al. : Immunosuppressive tryptophan catabolism and gut mucosal dysfunction following early HIV infection. J Infect Dis 2015;212:355–366 [DOI] [PubMed] [Google Scholar]
- 58.Routy JP, Mehraj V, Vyboh K, Cao W, Kema I, Jenabian MA: Clinical relevance of kynurenine pathway in HIV/AIDS: An immune checkpoint at the crossroads of metabolism and inflammation. AIDS Rev 2015;17:96–106 [PubMed] [Google Scholar]
- 59.Deminice R, Silva TC, de Oliveira VH: Elevated homocysteine levels in human immunodeficiency virus-infected patients under antiretroviral therapy: A meta-analysis. World J Virol 2015;4:147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]