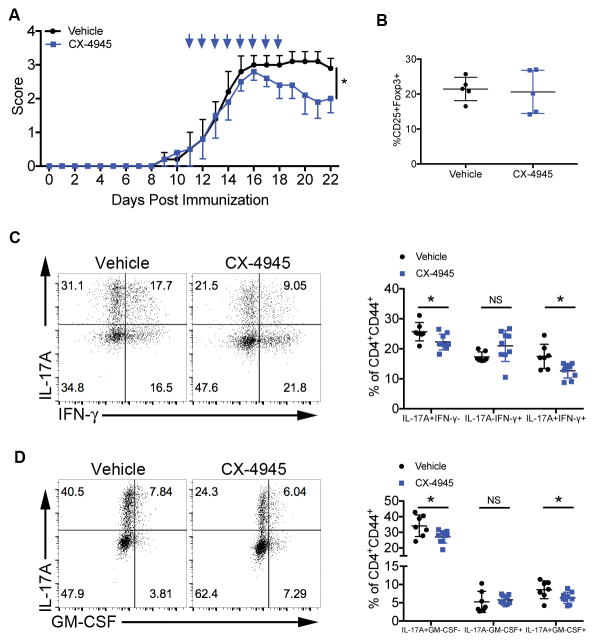
Figure 6. CX-4945 Therapeutic Treatment Suppresses Th17 Cell Differentiation and Reduces Disease Severity During EAE.
(A, B) Mice were treated with vehicle or CX-4945 via oral gavage for 8 days beginning at the onset of clinical symptoms. Data are from 1 representative experiment of at least 2 independent experiments. (A) Clinical scores from day 0 to 22 PI are shown; n=5/group. (B) At day 22, during disease resolution, mononuclear cells were enriched from the spinal cord and Tregs identified by CD25 and Foxp3 staining; n=5/group. (C, D) Mice were treated with vehicle or CX-4945 beginning on day 7 PI. At disease peak, effector T cells from the spinal cords were stained for (C) IL-17A and IFN-γ or (D) IL-17A and GM-CSF. Representative flow plots and frequencies of single and double positive cells +/− SEM are shown. Data were pooled from 3 independent experiments; vehicle, n=6; CX-4945, n=8. *p<0.05.