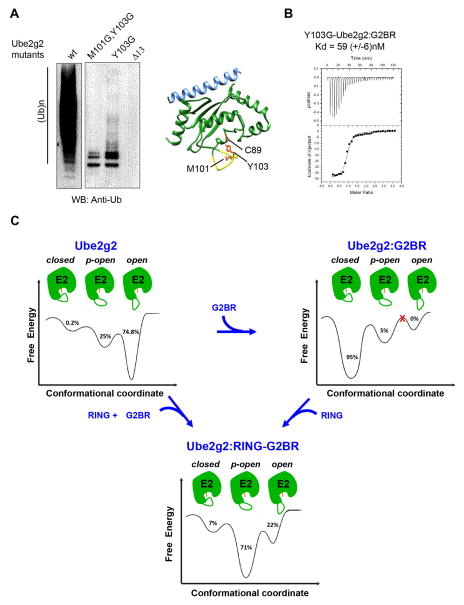
Figure 7. Functional role and dynamics of the gating loop.
(A) Reduced catalytic efficiency observed as changes in auto-ubiquitination of gp78c for different β4α2LL loop mutants in Ube2g2 compared to wt-Ube2g2. The Ube2g2:G2BR complex is depicted as Ube2g2 (green), G2BR (blue), and residues C89 (active site), M101 and Y103 shown as orange sticks. The Δ13 segment is shown in yellow. (B) ITC measurement of the interaction between G2BR and Y103G-Ube2g2. Kd = 59 ± 6 nM, stoichiometry 1:0.95, ΔH = −39.5 ± 0.4 kCal mol−1, and ΔS = −99.3 ± 0.5 Cal mol−1 K−1. (C) Dynamic energy landscape model of Ube2g2 and the redistribution of population among conformations in different gp78 domain bound states. The different conformations of Ube2g2, marked as either open, partially-open (p-open) or closed, were obtained from MD simulations performed at 298 K (Figure 5B). The MD trajectories sampled the different conformers multiple times within the duration of the simulation. The populations were obtained by binning the frames from MD runs into either the open, p-open or closed conformations. Ube2g2 is shown in green with active site C89 indicated in orange and the β4α2LL loop drawn as a green curve. See also Figure S4.