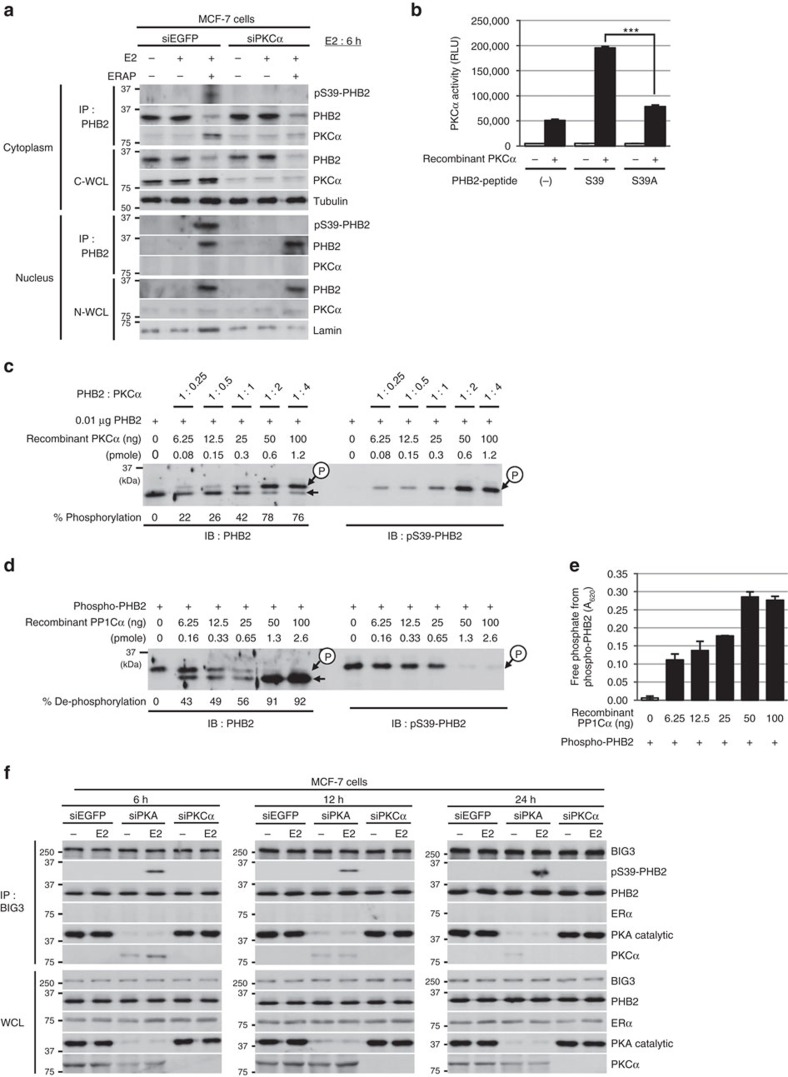
Figure 3. PHB2 is activated by phosphorylation at Ser39 via PKCα.
(a) The inhibitory effects of siPKCα on PHB2 phosphorylation at S39 in PHB2 immunoprecipitates of cytoplasmic and nuclear fractions in MCF-7 cells. C-WCL, total cytoplasmic lysates; N-WCL, total nuclear lysates. (b) In vitro PKCα activation assay of PHB2 using engineered peptides representing S39 and the alanine mutant of S39 (S39A). These data represent the means±s.e.m. of three independent experiments. (c) Phosphorylation at S39 in the recombinant full-length PHB2 by PKCα using Phos-tag SDS–PAGE and western blot analysis with anti-PHB2 and anti-pS39-PHB2 antibodies. (d) Dephosphorylation of full-length PHB2 phosphorylation at S39 by PP1Cα using Phos-tag SDS–PAGE and western blot analysis with anti-PHB2 and anti-pS39-PHB2 antibodies. (e) Phosphatase activity of PP1Cα against purified, recombinant full-length PHB2 phosphorylation at S39. These data represent the means±s.e.m. of three independent experiments. (f) The effects of siPKA and siPKCα on BIG3–PHB2 complexes and PHB2 S39-phosphorylation in MCF-7 cells after E2 stimulation for 6 h (left), 12 h (middle) and 24 h (right). ***P<0.001 (two-sided Student’s t-test).