Abstract
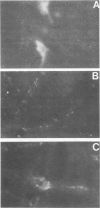
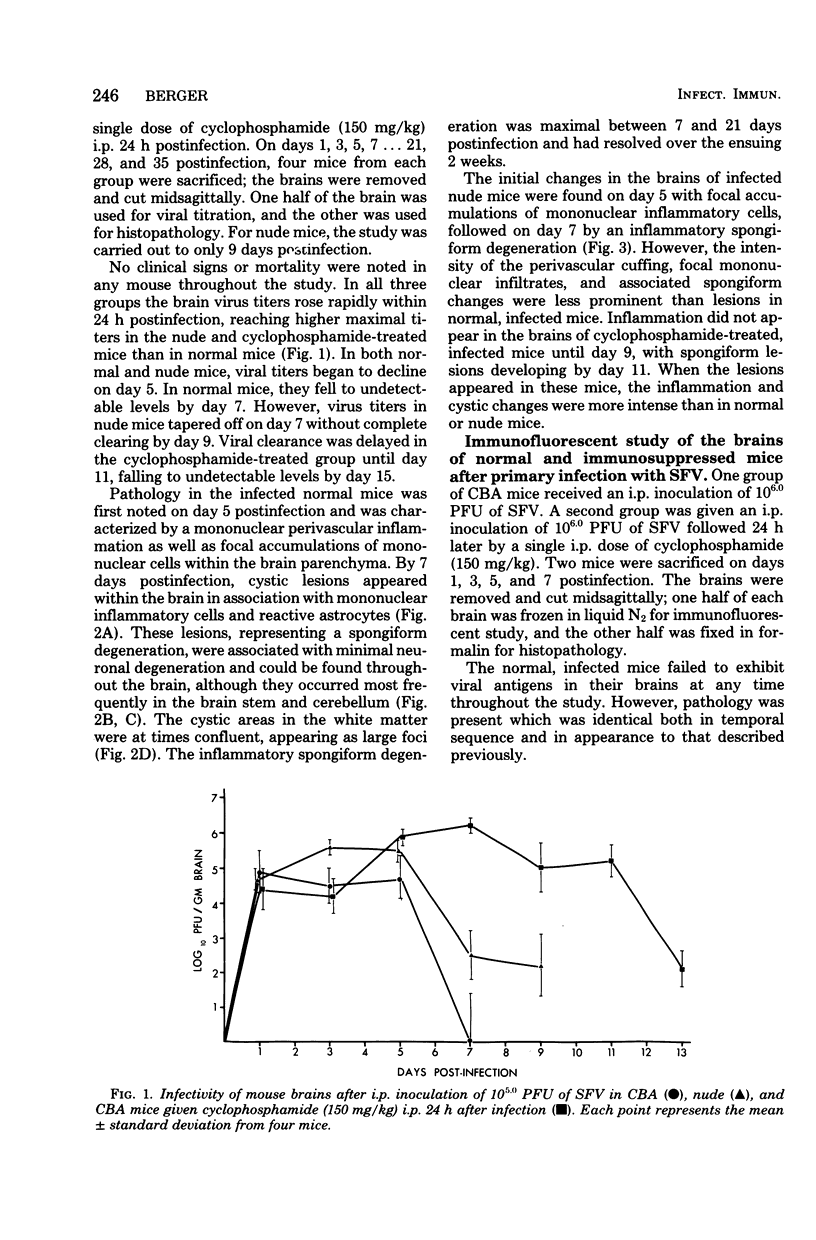
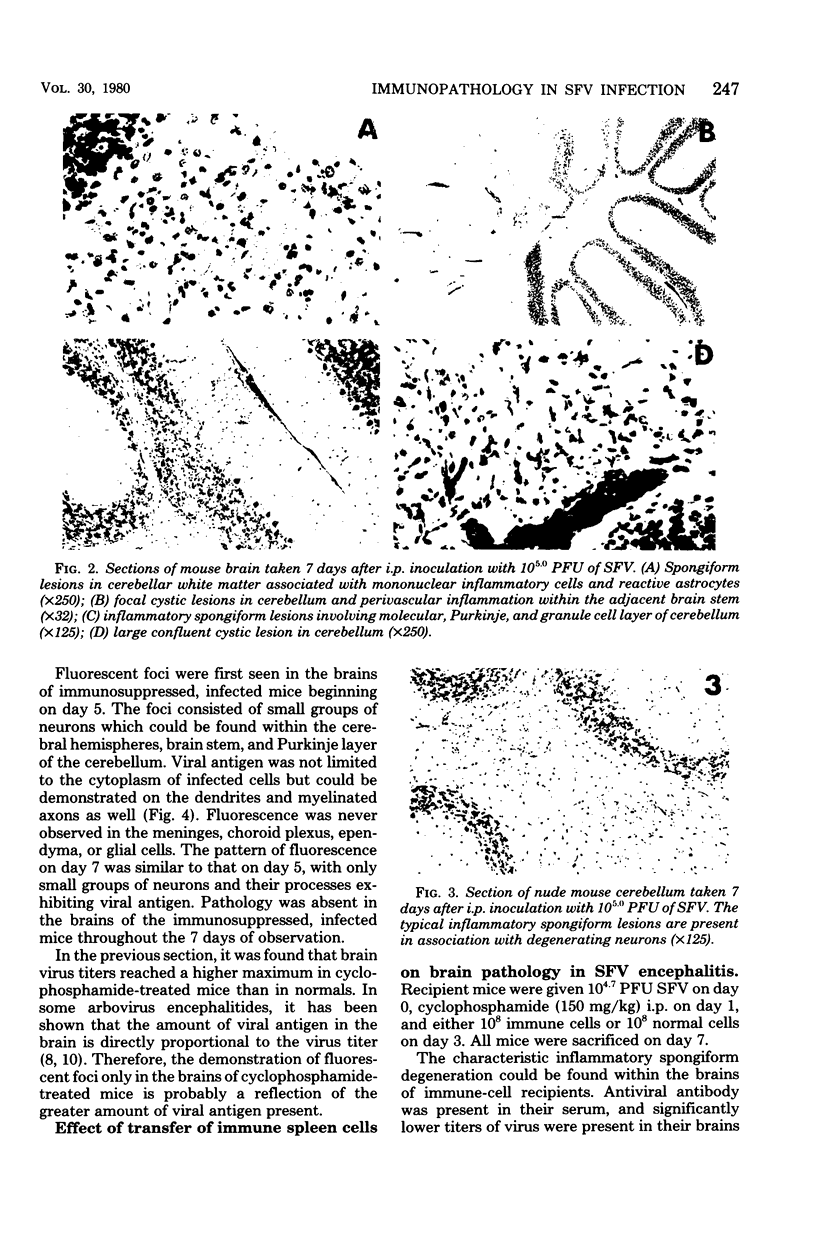
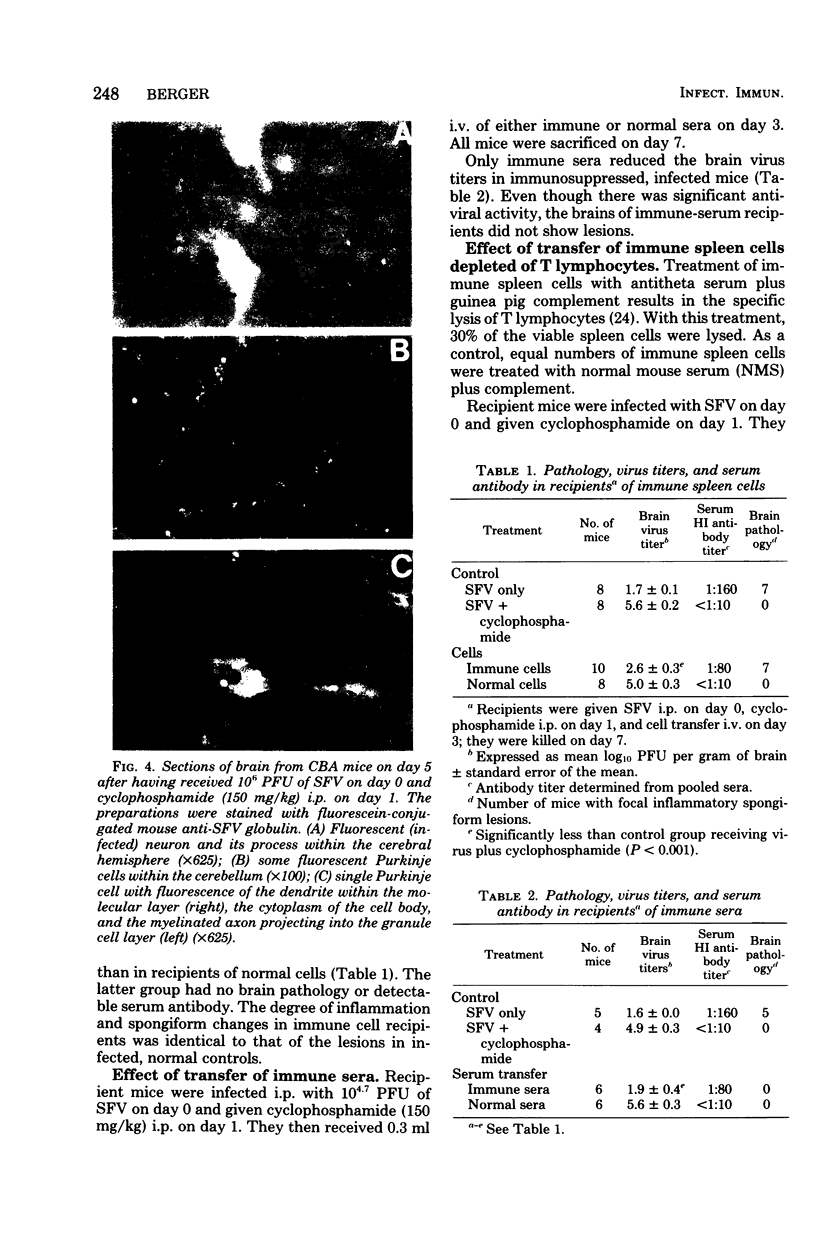
Seven days after peripheral inoculation with an avirulent strain of Semliki Forest virus, the brains of CBA and nude mice exhibited a mononuclear inflammation and spongiform degeneration. Mice that had received cyclophosphamide (150 mg/kg) 24 h after infection showed no pathology until day 11. However, immunofluorescence studies of the brains of immunosuppressed, infected mice demonstrated viral antigen within the soma and processes of neurons at earlier periods. The brain lesions could be reconstituted on day 7 in immunosuppressed, infected recipients with 6-day immune spleen cells. Immune spleen cells depleted of T lymphocytes, the non-immunoglobulin-bearing population deficient in B lymphocytes, or immune sera plus nonimmune bone marrow cells could also reconstitute the lesions. However, inflammation and spongiform changes were reduced when donor immune cells were depleted of either T or B lymphocytes. When both T and B lymphocytes were removed from the donor immune population, recipient brains did not show pathology. The results demonstrate that either antibody or immune T cells can trigger pathology, but there is also participation of nonimmune bone marrow-derived mononuclear cells, probably of the monocyte-macrophage lineage.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Nun A., Maron R., Ron Y., Cohen I. R. H-2 gene products influence susceptibility of target thyroid gland to damage in experimental autoimmune thyroiditis. Eur J Immunol. 1980 Feb;10(2):156–159. doi: 10.1002/eji.1830100216. [DOI] [PubMed] [Google Scholar]
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. 3. Regression infectious foci. J Exp Med. 1971 May 1;133(5):1090–1104. doi: 10.1084/jem.133.5.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. I. The effects of anti-thymocyte serum. J Exp Med. 1970 Nov;132(5):1035–1054. doi: 10.1084/jem.132.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradish C. J., Allner K., Maber H. B. The virulence of original and derived strains of Semliki forest virus for mice, guinea-pigs and rabbits. J Gen Virol. 1971 Aug;12(2):141–160. doi: 10.1099/0022-1317-12-2-141. [DOI] [PubMed] [Google Scholar]
- Bradish C. J., Fitzgeorge R., Titmuss D., Baskerville A. The responses of nude-athymic mice to nominally avirulent togavirus infections. J Gen Virol. 1979 Mar;42(3):555–566. doi: 10.1099/0022-1317-42-3-555. [DOI] [PubMed] [Google Scholar]
- Burns W., Billups L. C., Notkins A. L. Thymus dependence of viral antigens. Nature. 1975 Aug 21;256(5519):654–656. doi: 10.1038/256654a0. [DOI] [PubMed] [Google Scholar]
- CLARKE D. H., CASALS J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958 Sep;7(5):561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- Camenga D. L., Nathanson N., Cole G. A. Cyclophosphamide-potentiated West Nile viral encephalitis: relative influence of cellular and humoral factors. J Infect Dis. 1974 Dec;130(6):634–641. doi: 10.1093/infdis/130.6.634. [DOI] [PubMed] [Google Scholar]
- Cole G. A., Wisseman C. L., Jr, Nathanson N. Pathogenesis of type 1 dengue virus infection in suckling, weaning and adult mice. II. Immunofluorescent and histological studies. J Comp Pathol. 1973 Apr;83(2):243–252. doi: 10.1016/0021-9975(73)90048-0. [DOI] [PubMed] [Google Scholar]
- FENNER F. Mouse-pox; infectious ectromelia of mice; a review. J Immunol. 1949 Dec;63(4):341–373. [PubMed] [Google Scholar]
- Gilden D. H., Cole G. A., Nathanson N. Immunopathogenesis of acute central nervous system disease produced by lymphocytic choriomeningitis virus. II. Adoptive immunization of virus carriers. J Exp Med. 1972 Apr 1;135(4):874–889. doi: 10.1084/jem.135.4.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden D., Rorke L. B., Wroblewska Z., Wellish M. Parainfluenza 1 (6/94) virus-induced white matter degeneration: enhancement by adoptive transfer of virus-sensitized immunocytes. Neurology. 1978 Oct;28(10):1004–1007. doi: 10.1212/wnl.28.10.1004. [DOI] [PubMed] [Google Scholar]
- Hapel A. J. The protective role of thymus-derived lymphocytes in arbovirus-induced meningoencephalitis. Scand J Immunol. 1975;4(3):267–278. doi: 10.1111/j.1365-3083.1975.tb02626.x. [DOI] [PubMed] [Google Scholar]
- Hapel A., Gardner I. Appearance of cytotoxic T cells in cerebrospinal fluid of mice with ectromelia virus-induced meningitis. Scand J Immunol. 1974;3(3):311–319. doi: 10.1111/j.1365-3083.1974.tb01262.x. [DOI] [PubMed] [Google Scholar]
- Kindred B. Nude mice in immunology. Prog Allergy. 1979;26:137–238. [PubMed] [Google Scholar]
- Lay W. H., Nussenzweig V. Receptors for complement of leukocytes. J Exp Med. 1968 Nov 1;128(5):991–1009. doi: 10.1084/jem.128.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. Theiler's virus-induced demyelination: prevention by immunosuppression. Science. 1976 Apr 2;192(4234):62–64. doi: 10.1126/science.176726. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. II. Relationship of the anti-lymphocytic choriomeningitis immune response to tissue injury in chronic lymphocytic choriomeningitis disease. J Exp Med. 1970 Jan 1;131(1):1–19. doi: 10.1084/jem.131.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson G. R. In vitro and in vivo investigations on antibody-dependent cellular cytotoxicity. Curr Top Microbiol Immunol. 1978;80:65–96. doi: 10.1007/978-3-642-66956-9_3. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Suckling A. J., Jagelman S., Webb H. E. Brain lysosomal glycosidase activity in immunosuppressed mice infected with avirulent Semliki forest virus. Infect Immun. 1977 Feb;15(2):386–391. doi: 10.1128/iai.15.2.386-391.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomazic V., Rose N. R. Autoimmune murine thyroiditis VII: induction of the thyroid lesions by passive transfer of immune serum. Clin Immunol Immunopathol. 1975 Nov;4(4):511–518. doi: 10.1016/0090-1229(75)90092-6. [DOI] [PubMed] [Google Scholar]