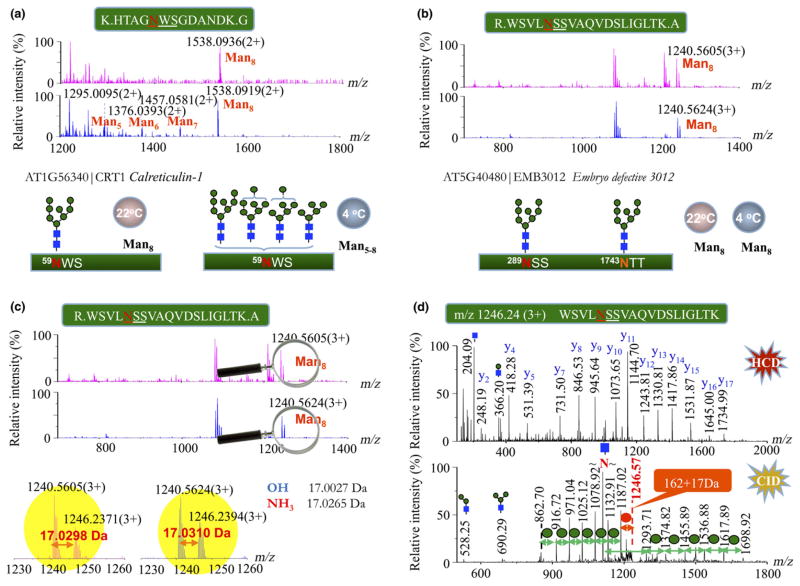
Fig. 4.
Discriminating the N-glycan profiles of endoplasmic reticulum (ER)-resident glycoproteins in plants under normal and cold-treated conditions for 18 d. (a) The mass spectra show the distinguishable high-mannose type N-glycans of the stress-responsive calreticulin-1 under two different conditions of plant growth: Man8GlcNAc2 (upper panel, 22°C) vs Man5-8GlcNAc2 (lower panel, 4°C). (b) Similar N-glycan profiles of Man8GlcNAc2 in the spectra were observed at the two glycosylation sites of Asn289 and Asn1743 of EMB3012 in plants grown under both conditions. The expanded m/z range of 1230–1260 from (b) showed the exact mass difference of 17.0299 ± 0.0001 Da between two peptide ions (c) at m/z 1240.56 (3+) and m/z 1246.24 (3+), which is close to that calculated from ammonium. (d) MS/MS spectra of the peptide precursor ion at m/z 1246.24 identified the same peptide sequence as the peptide of m/z 1240.56 at the higher-energy collision-induced dissociation (HCD, upper panel), and the additional modification of 17 Da was localized at the terminal mannose by the low-energy collision-induced dissociation (CID, lower panel) according to the initial neutral loss of 179 Da at m/z 1187.02 ([M + 3H-59.55]3+), that is, the 17 Da adduct of mannose from the precursor ion.