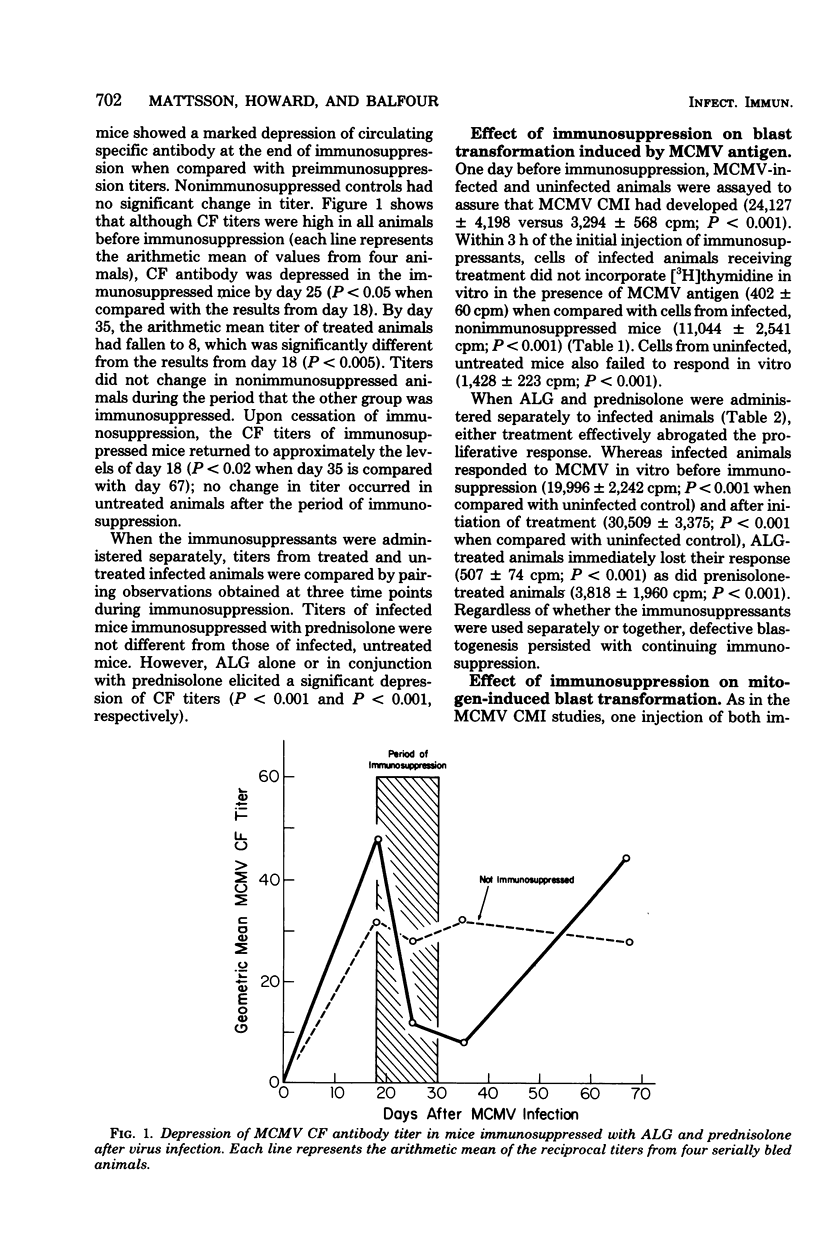
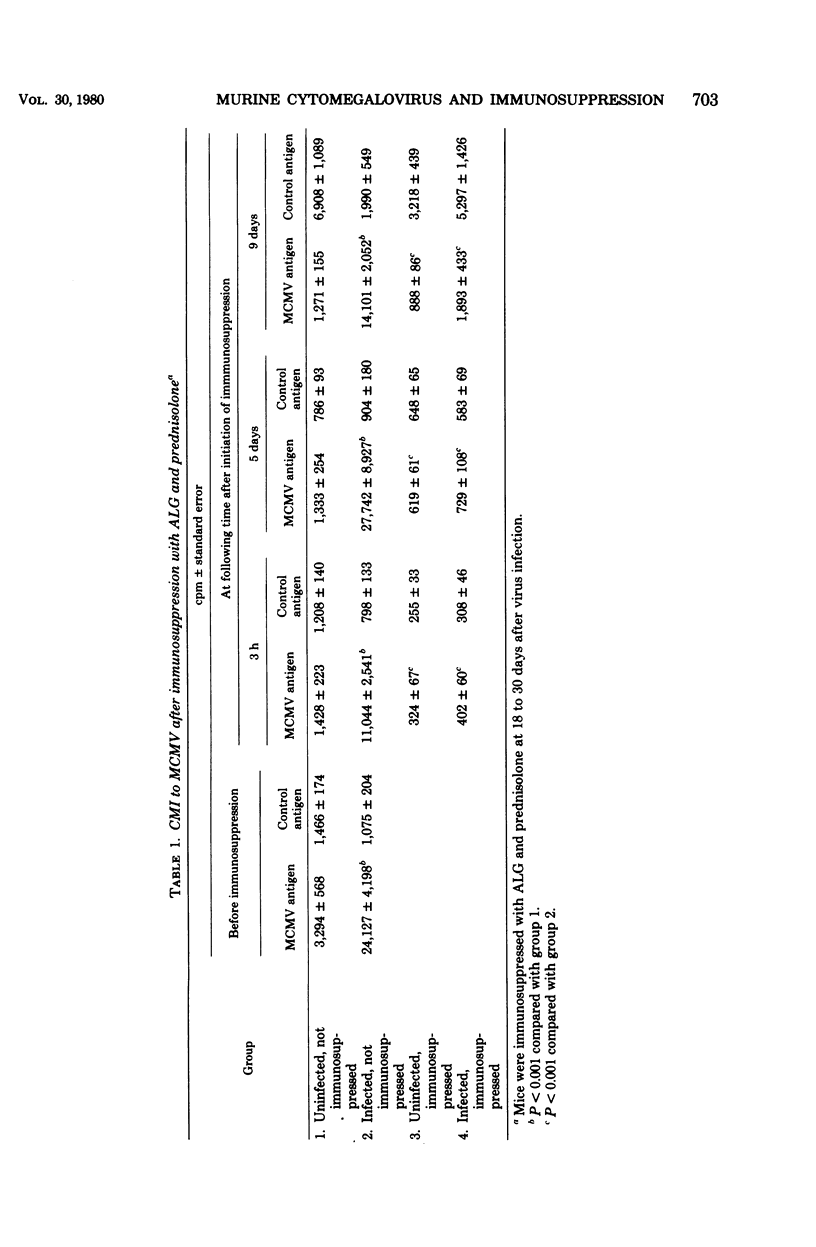
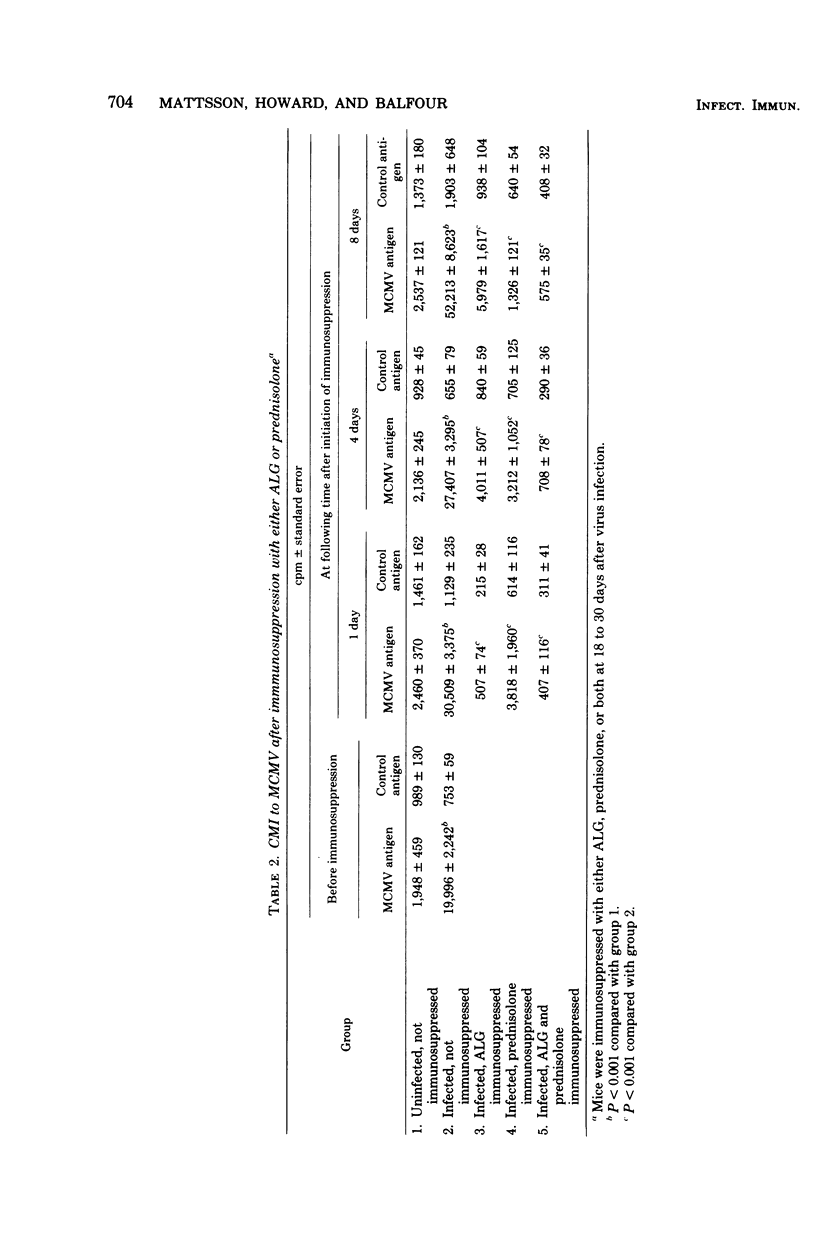
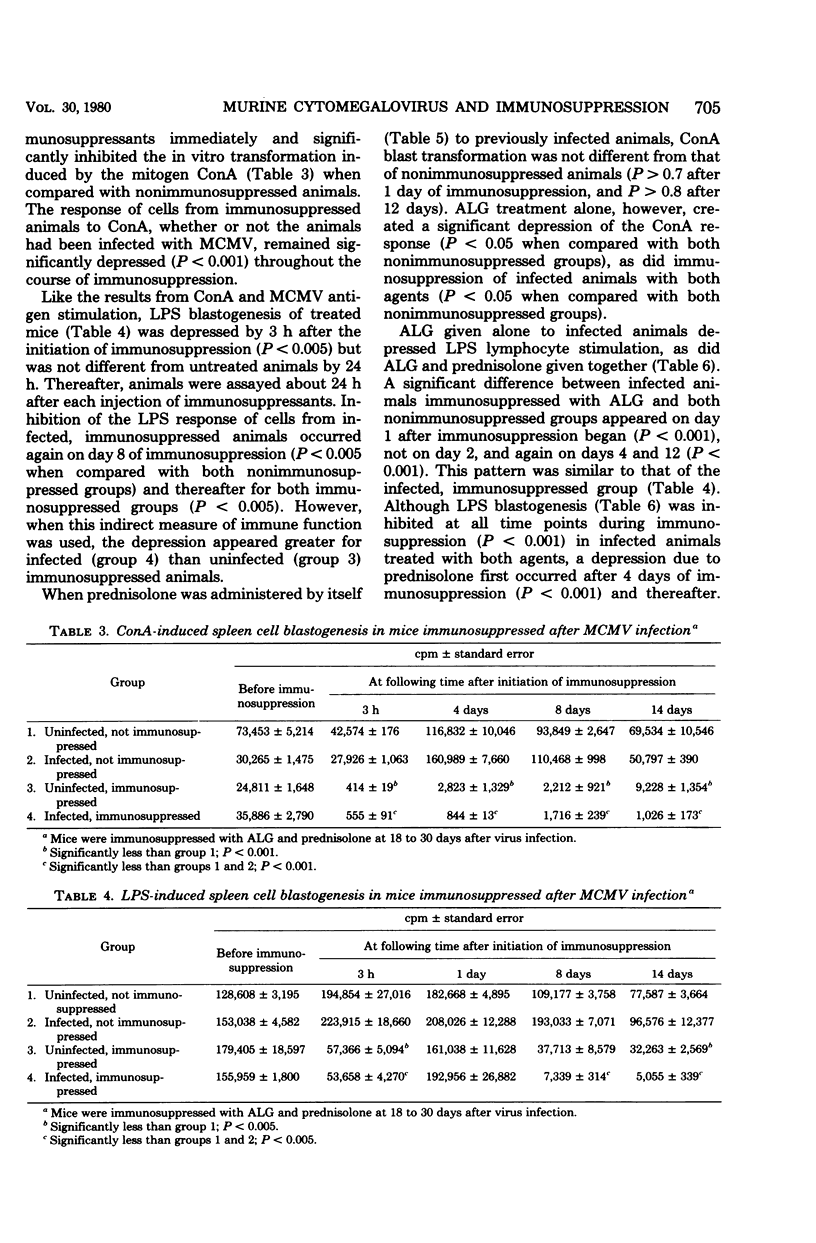
Abstract
Splenic lymphocytes from cytomegalovirus-infected mice lost their in vitro proliferative responses to cytomegalovirus antigen within 3 h after in vivo treatment with antilymphocyte globulin and prednisolone. The response was inhibited when the agents were administered separately or together, and inhibition persisted through a 2-week course of immunosuppression. Circulating specific antibodies were depressed by multiple injections of antilymphocyte globulin alone or with prednisolone, but not by prednisolone alone. Mitogen-induced blast transformation was immediately depressed by immunosuppression with both agents. Although the response to lipopolysaccharide returned briefly, it declined with continuing treatment. Cytomegalovirus infection augmented the depressive effect of immunosuppression on the lipopolysaccharide proliferative response. Prednisolone treatment of infected animals did not affect the concanavalin A response, and lipopolysaccharide stimulation decreased more slowly and to a lesser extent than it did in mice treated with antilymphocyte globulin or both agents. Loss of specific cell-mediated immunity and simultaneous depression of humoral immunity indicated that immunosuppression immediately created an inability to respond to an active cytomegalovirus infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balfour H. H., Jr, Slade M. S., Kalis J. M., Howard R. J., Simmons R. L., Najarian J. S. Viral infections in renal transplant donors and their recipients: a prospective study. Surgery. 1977 May;81(5):487–492. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- David D. S., Millian S. J., Whitsell J. C., Schwartz G. H., Riggio R. R., Stenzel K. H., Rubin A. L. Viral syndromes and renal homograft rejection. Ann Surg. 1972 Feb;175(2):257–259. doi: 10.1097/00000658-197202000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M. Role of specific cytotoxic lymphocytes in cellular immunity against murine cytomegalovirus. Infect Immun. 1980 Mar;27(3):767–776. doi: 10.1128/iai.27.3.767-776.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Balfour H. H., Jr Cell-mediated immunity to cytomegalovirus in mice and in renal transplant recipients. Transplant Proc. 1979 Mar;11(1):75–78. [PubMed] [Google Scholar]
- Howard R. J., Mattson D. M., Balfour H. H., Jr Effect of immunosuppression on humoral and cell-mediated immunity to murine cytomegalovirus. Proc Soc Exp Biol Med. 1979 Jul;161(3):341–346. doi: 10.3181/00379727-161-40549. [DOI] [PubMed] [Google Scholar]
- Howard R. J., Najarian J. S. Cytomegalovirus-induced immune suppression. I. Humoral immunity. Clin Exp Immunol. 1974 Sep;18(1):109–118. [PMC free article] [PubMed] [Google Scholar]
- Kelsey D. K., Overall J. C., Jr, Glasgow L. A. Correlation of the suppression of mitogen responsiveness and the mixed lymphocyte reaction with the proliferative response to viral antigen of splenic lymphocytes from cytomegalovirus-infected mice. J Immunol. 1978 Aug;121(2):464–470. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Linnemann C. C., Jr, Kauffman C. A., First M. R., Schiff G. M., Phair J. P. Cellular immune response to cytomegalovirus infection after renal transplantation. Infect Immun. 1978 Oct;22(1):176–180. doi: 10.1128/iai.22.1.176-180.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notkins A. L. Immune mechanisms by which the spread of viral infections is stopped. Cell Immunol. 1974 Mar 30;11(1-3):478–483. doi: 10.1016/0008-8749(74)90045-8. [DOI] [PubMed] [Google Scholar]
- Pollard R. B., Rand K. H., Arvin A. M., Merigan T. C. Cell-mediated immunity of cytomegalovirus infection in normal subjects and cardiac transplant patients. J Infect Dis. 1978 May;137(5):541–549. doi: 10.1093/infdis/137.5.541. [DOI] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Black P. H., Hirsch M. S. Interaction of cytomegalovirus with leukocytes from patients with mononucleosis due to cytomegalovirus. J Infect Dis. 1977 Nov;136(5):667–678. doi: 10.1093/infdis/136.5.667. [DOI] [PubMed] [Google Scholar]
- Rytel M. W. Humoral and cell-mediated immunity to cytomegaloviruses. Yale J Biol Med. 1976 Mar;49(1):63–63. [PMC free article] [PubMed] [Google Scholar]
- Simmons R. L., Lopez C., Balfour H., Jr, Kalis J., Rattazzi L. C., Najarian J. S. Cytomegalovirus: Clinical virological correlations in renal transplant recipients. Ann Surg. 1974 Oct;180(4):623–634. doi: 10.1097/00000658-197410000-00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock E. F., Toy S. T. Participation of lymphocytes in viral infections. Adv Immunol. 1973;16:123–184. doi: 10.1016/s0065-2776(08)60297-7. [DOI] [PubMed] [Google Scholar]


