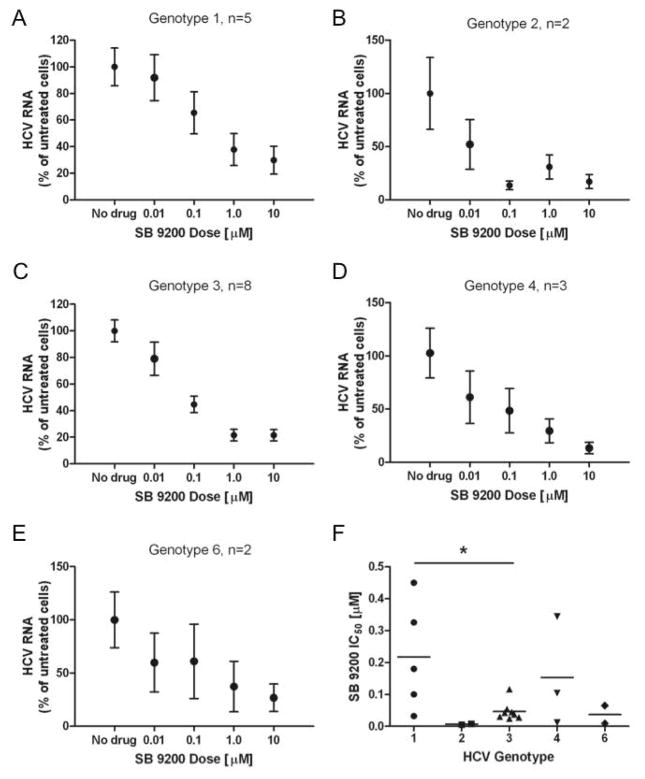
Figure 1. SB 9200 demonstrates pan-genotypic antiviral activity against HCV.
The sensitivity of patient derived HCV to SB 9200 was assessed in the phenotypic capture fusion assay. HCV-containing fused cells were treated with varying concentrations of SB 9200 and HCV RNA was quantified. Pooled results, normalised to untreated cells to account for variability in HCV RNA yield between samples from different donors are shown for each genotype (A–E) values are mean ± s.e.m. Comparison of the individual IC50 for SB 9200 across the HCV genotypes tested (F) p value (*=p<0.05) was calculated using Mann Whitney U test