Figure 8.
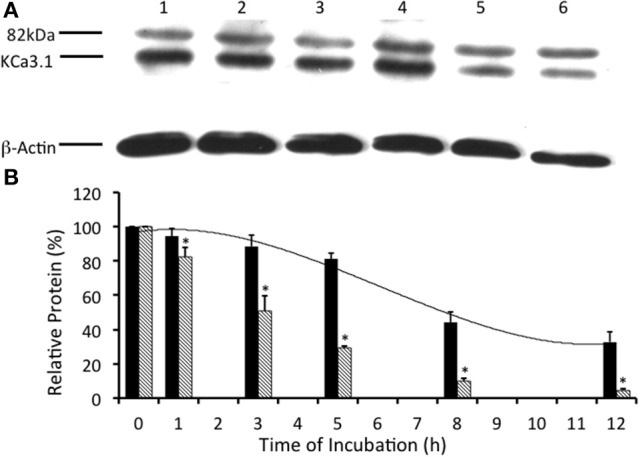
Effect of lysosomal inhibitors on the streptavidin-labeled membrane bound KCa3.1-BLAP channels. Lysosomal activities were inhibited with leupeptin (100 μM, s and m) and pepstatin (1 μg/ml, s and m; L/P) to determine the effect on the degradation of KCa3.1. (A) The degradation time course was executed with the addition of leupeptin and pepstatin after streptavidin labeling of KCa3.1. After labeling, cells were further incubated in 37° C for 0 (no incubation), 1, 3, 5, 8, or 12 h with L/P before cells were lysed and detection of KCa3.1 by immunoblot. Lane 1: Control no incubation (t = 0), Lane 2: 1 h of incubation (t = 1), Lane 3: 3 h of incubation (t = 3), Lane 4: 5 h of incubation (t = 5), Lane 5: 8 h of incubation (t = 8), and Lane 6: 12 h of incubation (t = 12). Each lane was loaded with 30 μg of protein and β-actin was used as a loading control. (B) Immunoblot results were quantified by densitometry. As seen, in the presence of L/P, the degradation of KCa3.1 (black bars) was greatly reduced, retaining high levels of KCa3.1 expressed even at 5 h after labeling compared with 5 h non-treated control cells (hatched bars) shown from Figure 2 (*P ≤ 0.05, n = 5). In the presence of L/P, the relative protein expression of KCa3.1 exhibited a half-life that was increased by ~4 h compared to normal control degradation data (Figure 2).
