Abstract
Purpose
The aim of this study was to determine the effect of single fraction (SF) and multiple fraction (MF) radiation therapy (RT) on bone mineral density (BMD) in patients with cancer and bone metastases in the proximal femur. We studied this effect in the radiation field and within metastatic lesions, and differentiated between lytic, blastic, and mixed lesions.
Methods and materials
This prospective cohort study comprised 42 patients with painful bone metastases, including 47 irradiated femora with 52 metastatic lesions in the proximal femur. Patients received either 8 Gy SF or 20 to 24 Gy in 5 to 6 fractions (MF). Quantitative computed tomography scans were obtained before RT and 4 and 10 weeks after the initial scan. Patients who received MF additionally underwent quantitative computed tomography on the final day of their treatment. Automated image registration was performed. Mean BMD was determined at each time point for each proximal femur (region of interest [ROI]-PF) and in greater detail for a region of interest that contained the metastatic lesion (ROI-ML). Statistical analysis was performed using linear mixed models.
Results
No significant differences in mean BMD were found between SF or MF RT over all time points in both ROI-PF and ROI-ML. Mean BMD did not change in ROI-PF with lytic and mixed lesions, but mean BMD in ROI-PF with blastic lesions increased to 109%. Comparably, when focused on ROI-ML, no differences in mean BMD were observed in lytic ROI-ML but mean BMD in mixed and blastic ROI-ML increased up to 105% and 121%, respectively.
Conclusions
Ten weeks after palliative radiation therapy in patients with femoral metastatic lesions, a limited increase in BMD was seen with no beneficial effect of MF over SF RT. BMD in lytic lesions was unchanged but slightly increased in mixed and blastic lesions.
Summary.
The effect of palliative radiation therapy on the bone mineral density (BMD) of femoral bone metastases and the surrounding bone was studied in 42 patients with quantitative computed tomography scans. Ten weeks after treatment, no difference in BMD was seen between patients who were treated with single or multiple fractions. BMD did not change in lytic lesions but increased slightly in mixed and blastic lesions over time.
Introduction
Bone metastases are most frequently seen in patients with primary tumors in the breast, prostate, lung, kidney, or thyroid.1, 2 These lesions can have a lytic, blastic, or mixed radiological appearance. Lytic lesions result from disproportionate bone resorption by osteoclasts and leads to progressive destruction of the bone tissue and a subsequent risk of pathological fracturing.1, 3 Blastic lesions are characterized by excessive bone formation and are hypothesized to decrease bone strength because the newly formed bone has a reduced structural integrity.1, 3
External beam radiation therapy (RT) plays an important role in the palliative care of patients with bone metastases because it reliefs pain.4, 5, 6, 7 Additionally, some studies report a beneficial effect of RT on bone mineral density (BMD),8, 9, 10, 11, 12 which is also the clinical experience of medical specialists. In contrast, this beneficial effect was not confirmed in a recent systematic review.13 Moreover, the relationship between pain relief and BMD is unclear. Some authors suggest there is no relationship,7, 11 whereas others state that improved BMD contributes to long-lasting pain relief,14 increased bone stability, and decreased risk of fractures.15
In patients with cancer and bone metastases in the femur, the ability to walk and remain mobile is very important for the overall quality of life. Therefore, it is important to assess not only pain but also the risk for fractures when determining RT dose schedules. If the expected risk for fractures is low, RT is administered in one, relatively high dose (single fraction [SF]) to relieve pain. Patients with a high expected risk for fractures who are not eligible for or do not want surgery can receive RT in multiple fractions (MF) to induce remineralization and prevent pathological fracturing.16, 17 Previous research suggested that 24 Gy in 4 fractions postponed pathological fractures when compared with a single dose of 8 Gy.17 However, studies on the effect of RT doses on BMD are limited, and the reported responses differ between studies.8, 9, 10, 11, 12 To date, only Koswig et al compared SF and MF in terms of BMD and found a greater response after MF RT.8
Most studies included an analysis of lytic lesions in the vertebrae8, 9, 10, 11 but the effect of RT on BMD in blastic or mixed lesions was often not considered. Although the femur is also frequently affected,18, 19 few studies analyzed the effect of RT on BMD in femoral lesions.8, 9, 12 Also, little is known about the effect of RT on BMD within the entire field of RT as it relates the femur.
Therefore, the aim of this study is to determine the effect of SF and MF RT on BMD in patients with cancer and femoral bone metastases. Specifically, we studied 2 regions of interest: the proximal femur within the radiation field (region of interest [ROI]-PF) and the metastatic lesion (ROI-ML). For both regions, we investigated whether there was an overall effect of SF and MF RT on BMD and whether these effects were different in the femora among lytic, blastic, and mixed lesions.
Methods and materials
Patients
Between 2006 and 2009, patients who received palliative RT for femoral metastases in 3 RT institutes in The Netherlands (Radiotherapeutic Institute Friesland, Leiden University Medical Center and Radboud University Medical Center) were asked to participate in this prospective study. Institutional approval was obtained from the ethics committees of all participating centers. This study is part of a larger study on the prediction of fracture risks with use of Finite Element modeling.20, 21
Patients received either SF (1 × 8 Gy) or MF (5 or 6 × 4 Gy) RT in accordance with the Dutch clinical guidelines that state that lesions with cortical involvement of more than 3 cm have an increased risk of fracture and will be considered for prophylactic surgery. 16, 17 SF is typically applied to treat pain that is related to smaller, uncomplicated lesions with a low expected risk for fracture and has a 60% to 80% chance of pain relief.4, 5, 6, 7 In patients who have larger lesions (ie, in principle requiring stabilization) and refuse surgery or have a deteriorating clinical condition, radiation oncologists typically prescribe a higher total dose to induce remineralization. Surgery may be too hazardous for these patients; hence, higher radiation doses are selected with the hope that they prevent pathologic fracturing.
Patients were included in the study if they had a Karnofsky Performance Score of >60, no clinical or radiologic evidence of pathologic femoral fractures, no planned or prior palliative surgery to the femur, no radionuclide treatment 30 days prior to inclusion, and no previous RT to the femur. In total, 66 patients gave written informed consent. With use of follow-up questionnaires after 4 and 10 weeks and after 4, 5, and 6 months, patients were actively followed for 6 months or until they sustained a femoral fracture or died. At the 6-month follow-up, the study database was updated on the basis of hospital records and then closed.
Measurements and follow-up
Baseline patient characteristics including sex, age, body weight, Karnofsky Performance Score, time since primary tumor diagnosis and since first metastasis, primary tumor, and concurrent systemic therapy were registered by the treating radiation oncologist at the time of intake. During the RT planning session, patients underwent their first quantitative computed tomography (QCT) scan. Subsequent QCT scans were taken after 4 and 10 weeks. Patients who received MF RT also underwent an additional QCT scan after 1 week on the final day of their RT schedule to determine a potential immediate effect of RT on BMD.8, 10 At the same time points, patients completed questionnaires on pain (ie, Brief Pain Inventory22), level of activity, and quality of life (including sections from the Longitudinal Aging Study Amsterdam Physical Activity Questionnaire,23 Short Form-36,24 and the Western Ontario and McMaster Universities Arthritis Index25). These patient-reported outcomes will be published separately.
The institutes were instructed to perform the QCT scans in accordance with a standardized protocol with the following settings: 120 kVp, 220 mA, slice thickness 3 mm, pitch 1.5, spiral and standard reconstruction, and in-plane resolution 0.9375 mm. The protocol required scanning of at least the proximal half of the femur, including the painful metastases. A solid calibration phantom (Image Analysis, Columbia, KY) that contained 4 known calcium hydroxyapatite (CaHA) concentrations of 0, 50, 100, and 200 mg/cm3 was placed under the patient in the scanner. The densities in this phantom were used to calibrate each Hounsfield Unit (HU) to CaHA density. This CaHA density is a calcium-equivalent density that is a measure of BMD; in the remainder of this work, we will refer to it as BMD.
Registration
To analyze the effect of RT on BMD of the proximal femur region (ROI-PF) over time, the proximal half of the femur for each patient was segmented from one of the QCT scans (Mimics 11.0 and 14.0, Materialise, Leuven, Belgium).
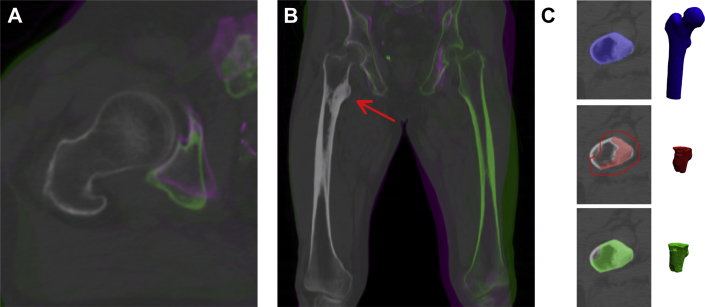
Patients' QCT scans were registered with fully automated rigid registration algorithms for medical images (elastix26, 27). For this, numerous alignments of 2 CT scans were calculated until the best fit was found,28 resulting in an objective and accurate registration (Fig. 1A and B). All voxels that represent the femoral geometry were included in the analyses.
Fig. 1.
Transversal (A) and sagittal (B) false color overlay of two registered quantitative computed tomography scans of the right femur, showing an accurate registration of the right femur (no green/purple color visible) and inaccurate registration of the pelvis and left femur (green/purple colors visible). The red arrow indicates the lesion. (C) Segmented geometry of a proximal femur (ROI-PF, blue), lesion (red), and 6-mm margin (line), and the region of interest that was used for analysis of the lesion (ROI-ML, green).
Furthermore, an experienced radiation oncologist segmented the lesions and scored each metastatic femur as lytic, blastic, or mixed on the first QCT scan. To account for obscure edges, regions of metastatic lesions (ROI-ML) were defined by expanding the lesions by 6 mm in all directions. If the procedure included voxels outside the femur, these voxels were ignored (Fig 1C).
Analysis
Baseline characteristics were compared between patients who received SF and MF and patients with lytic, blastic, and mixed lesions with use of the Mann-Whitney U, Fisher exact, Pearson χ2, and Kruskal-Wallis tests, where applicable.
Mean BMD in mg/cm3 was calculated for each ROI-PF and ROI-ML at each time point. Linear mixed models were used to study the effect of RT on the BMD of all ROI-PF and ROI-ML over time. This analysis allowed for the inclusion of patients with missing data. Lesion type and size were added to the models. To address a potential effect of confounding by indication, we tested whether other baseline characteristics (eg, performance score, primary tumor, concurrent systemic therapy [anticancer and/or bisphosphonates]) also affected BMD. However, none of these other baseline characteristics influenced the effect of RT on BMD, and they were removed from the final models.29
A random intercept was included to disregard the variability in initial BMD between patients. The interaction between lesion type and time was significant and therefore added to the model. All other interactions were not significant. P-values below .05 were considered statistically significant. Statistical analyses were performed using Stata/SE 11.2 (StataCorp LP, College Station, TX).
The statistical model tested BMD in mg/cm3 but not in percentages. However, for interpretational and visual purposes, the data were converted to percentages by marking BMD on the first QCT scan as 100% and calculating BMD of the subsequent QCT scans relative to the first measurement.
Results
Patients
Of the 66 eligible patients, 24 were excluded from this analysis because only 1 QCT scan was available (n = 20), the first QCT scan was missing (n = 1), or lesions were unidentifiable (n = 3). Hence, 42 patients were included for analysis, 5 of whom received RT to both femora, leading to a total of 47 femora for analysis. Three femora had 2 separate lesions and 1 femur had 3 lesions, which resulted in 52 lesions and comprised 24 lytic, 8 blastic, and 20 mixed lesions.
Not every patient underwent all QCT scans at all time points because of death, fracture, or deteriorating condition. At baseline, all 42 QCT scans were obtained, but after 4 and 10 weeks, only 30 and 27 QCT scans were acquired, respectively. Of the 26 patients who receive MF RT, 25 underwent a QCT scan on the final day of RT. Against protocol instructions, less than half of the femur was scanned in 12 cases (range, 41%-49% of the femoral length). Additionally, 8 ROI-PF were larger than 50% to include lesions in the distal half of the femur (range, 55%-89% of the femoral length).
Baseline characteristics were not significantly different between patients who received SF or MF RT, but sex, age, and primary tumor differed among patients with lytic, blastic, or mixed lesions (Table 1).
Table 1.
Patient baseline characteristics
| Single fraction (1 × 8 Gy) n = 16 |
Multiple fractions (5-6 × 4 Gy) n = 26 |
P-value | Lytic n = 17 |
Blastic n = 8 |
Mixed n = 17 |
P-value | |
|---|---|---|---|---|---|---|---|
| Sexa | 0.5 | 0.02 | |||||
| Male | 12 (75%) | 16 (62%) | 7 (41%) | 7 (87%) | 14 (82%) | ||
| Female | 4 (25%) | 10 (38%) | 10 (59%) | 1 (13%) | 3 (18%) | ||
| Age, yrb | 0.8 | 0.03 | |||||
| Median (Range) | 68.5 (39-89) | 66 (52-85) | 62 (39-84) | 69 (61-75) | 70 (46-89) | ||
| Body weight, kgb | 0.5 | 0.5 | |||||
| Median (Range) | 75 (44-92) | 79 (57-106) | 73 (57-106) | 77.5 (44-83) | 84.5 (56-95) | ||
| Karnofsky Performance Scoreb | 0.1 | 0.5 | |||||
| Median (Range) | 80 (60-100) | 80 (60-100) | 80 (60-90) | 80 (70-90) | 80 (60-100) | ||
| Time since primary tumor diagnosis, yrb | 0.6 | 0.3 | |||||
| Median (Range) | 4.0 (0.4-17.6) | 3.0 (0.1-23.8) | 1.9 (0.1-23.8) | 5.9 (0.2-12.3) | 2.8 (0.4-17.6) | ||
| Time since first metastasis, yrb | 0.8 | 0.3 | |||||
| Median (Range) | 1.2 (0-7.3) | 1.0 (0.3-7.8) | 0.8 (0-7.8) | 2.6 (0-7.3) | 1.2 (0-5.4) | ||
| Primary tumorc | 0.6 | 0.004 | |||||
| Breast | 3 (19%) | 5 (19%) | 5 (30%) | 1 (13%) | 2 (12%) | ||
| Lung | 1 (6%) | 5 (19%) | 4 (23%) | 1 (13%) | 1 (6%) | ||
| Prostate | 9 (56%) | 10 (38%) | 1 (6%) | 6 (74%) | 12 (70%) | ||
| Otherd | 3 (19%) | 6 (23%) | 7 (41%) | 0 (0%) | 2 (12%) | ||
| Lesion typec | 0.2 | ||||||
| Lytic | 4 (25%) | 13 (50%) | |||||
| Blastic | 3 (19%) | 5 (19%) | |||||
| Mixed | 9 (56%) | 8 (31%) | |||||
| Affected femura | 1 | 0.8 | |||||
| Unilateral | 14 (87%) | 23 (89%) | 14 (82%) | 8 (100%) | 15 (88%) | ||
| Bilateral | 2 (13%) | 3 (11%) | 3 (18%) | 0 (0%) | 2 (12%) | ||
| Concurrent systemic therapyc | 0.6 | 0.2 | |||||
| No concurrent therapy | 1 (6%) | 5 (19%) | 5 (29%) | 1 (13%) | 0 (0%) | ||
| Systemic therapy+Bisphosphonates | 4 (25%) | 6 (23%) | 3 (18%) | 1 (13%) | 6 (35%) | ||
| Systemic therapy–Bisphosphonates | 10 (63%) | 12 (46%) | 7 (41%) | 5 (61%) | 10 (59%) | ||
| Bisphosphonates only | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| Missing | 1 (6%) | 3 (12%) | 2 (12%) | 1 (13%) | 1 (6%) |
Tested with Fisher exact.
Tested with Mann-Whitney U for differences between single and multiple fractions and with Kruskal-Wallis for differences between lytic, blastic and mixed lesions.
Tested with Pearson χ2.
Other = Kidney, Rectum, Kahler's disease, Urethra, Cervix or aCUP (cancer of unknown primary origin).
One patient who received SF sustained a femoral fracture after 3 months. Of the patients who received MF, 1 patient fractured a femur after 2 weeks and 1 patient fractured both femora after 4 months.
Bone mineral density
Table e1 depicts the mean BMD (in mg/cm3) of the proximal femora (ROI-PF) and metastatic lesions (ROI-ML) from the scans that were available (Supplementary Material).
Effect of RT on BMD in proximal femur (ROI-PF)
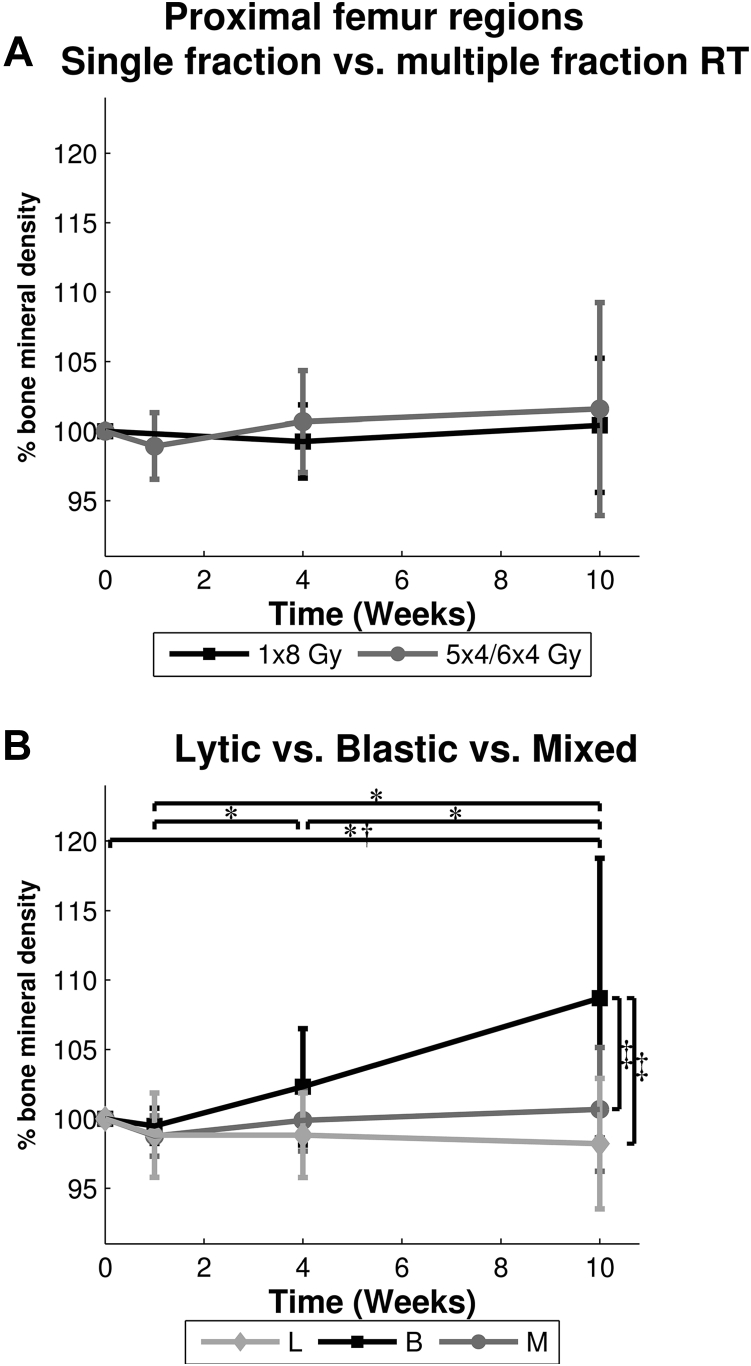
At baseline, mean BMD in ROI-PF was 471.5 mg/cm3 (standard deviation [SD], 77.4 mg/cm3) in patients who were assigned to SF RT and 445.7 mg/cm3 (SD, 70.7 mg/cm3) in patients who were assigned to MF RT. After 10 weeks, BMD increased 0% after SF and 2% after MF RT and was not different between SF and MF RT over all time points (Fig 2A). An interaction was found between lesion type and time, which indicates that femora affected by different lesion types responded diversely to RT over time (Fig 2B). This lesion-dependent effect over time holds for both radiation schedules, as there was no difference in BMD between ROI-PF treated with SF and MF RT.
Fig. 2.
Mean ± standard deviation bone mineral density (in percentage relative to quantitative computed tomography scan 1) of all proximal femur regions (ROI-PF) over time, for (A) single fraction (1 × 8 Gy) versus multiple fractions (5-6 × 4 Gy), and (B) each lesion type. *Significant difference for blastic lesions. †Significant difference for lytic lesions. ‡Significant difference at 10 weeks.
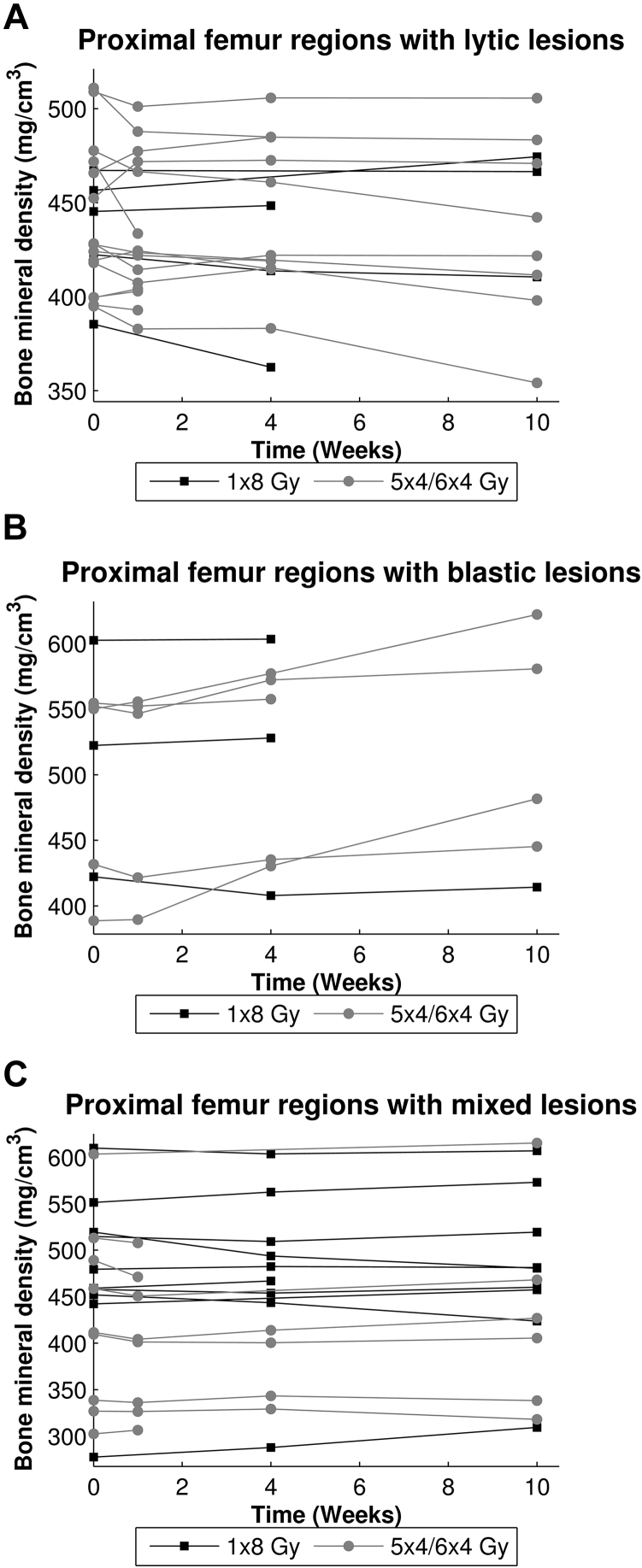
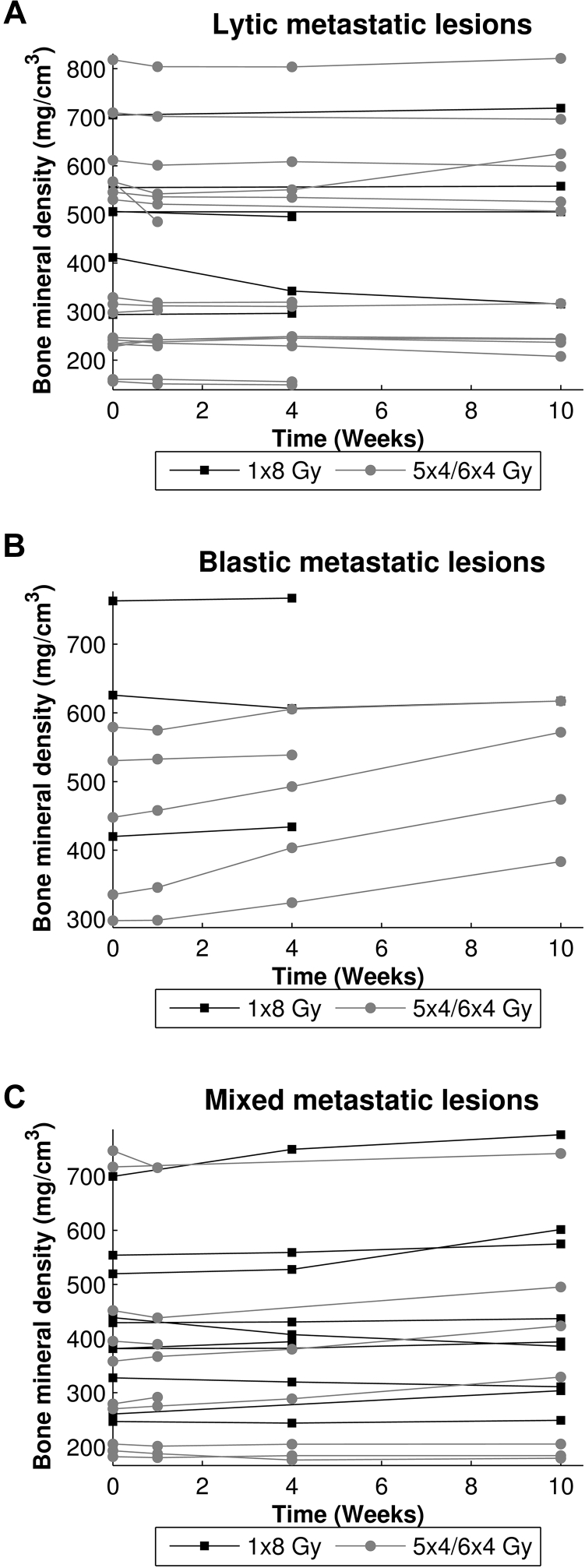
Independent of RT schedule, ROI-PF that included lytic lesions showed a significant decrease of 2% in BMD between QCT scans at baseline and after 10 weeks, (−9.2 mg/cm3, 95% confidence interval [CI], −18.0-−0.4, P = .04). An increase in BMD to 109% at 10 weeks (37.9 mg/cm3, 95% CI 24.7-51.0, P < .001) was observed in ROI-PF that contained blastic lesions. No significant differences over time were found for ROI-PF with mixed lesions. Furthermore, 10 weeks after RT, BMD in ROI-PF that contained blastic lesions was significantly higher than in ROI-PF that contained lytic (106.4 mg/cm3, 95% CI 39.1-173.7, P = .002) or mixed lesions (87.2 mg/cm3, 95% CI 24.8-149.6, P = .006). Figure 3 depicts the BMD of all ROI-PF and shows a widespread individual response to RT.
Fig. 3.
Mean bone mineral densities (in mg/cm3) of the proximal femur regions (ROI-PF) that contain lytic (A), blastic (B), or mixed (C) lesions for each femur over time.
Effect of RT on BMD in metastatic lesion (ROI-ML)
On average, ROI-ML volume was 88 cm3 (SD, 61 cm3). Volumes of lytic (mean, 58 cm3; SD, 44 cm3), blastic (mean, 143 cm3; SD, 78 cm3), and mixed (mean, 103 cm3; SD, 53 cm3) ROI-ML were significantly different (P = .004).
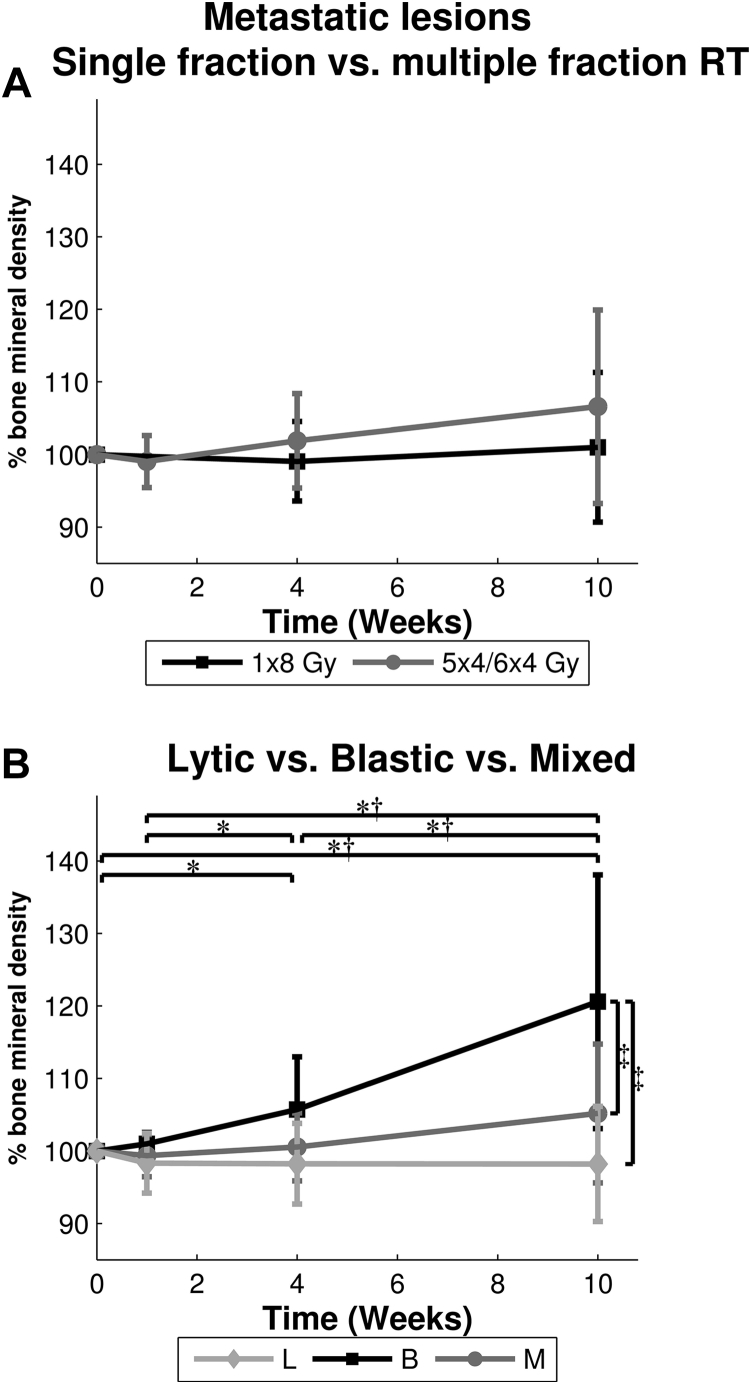
At baseline, the mean BMD in ROI-ML treated with SF RT was 474.9 mg/cm3 (SD, 150.1 mg/cm3), and 394.6 mg/cm3 (SD, 190.3 mg/cm3) when treated with MF RT. Mean BMD of ROI-ML increased by 1% in SF and 7% in MF RT after 10 weeks, but this was not significantly different (Fig 4A). A significant interaction between lesion type and time was found, indicating that the effect of RT on BMD was different for the 3 lesion types (Fig 4B).
Fig. 4.
Mean ± standard deviation bone mineral density (in percentage relative to quantitative computed tomography scan 1) of the metastatic lesions (ROI-ML) over time for (A) single fraction (1 × 8 Gy) versus multiple fractions (5-6 × 4 Gy), and (B) each lesion type. *Significant difference for blastic lesions. †Significant difference for mixed lesions. ‡Significant difference at 10 weeks.
No changes in BMD were observed in lytic ROI-ML over time. BMD in blastic and mixed ROI-ML significantly increased by 21% and 5%, respectively, between baseline and 10 weeks, (blastic: 72.8 mg/cm3, 95% CI 50.5-95.0, P < .001; mixed: 22.0 mg/cm3, 95% CI 9.2-34.9, P = .001).
At baseline, BMD in blastic ROI-ML was significantly higher than in lytic (161.4 mg/cm3; 95% CI, 6.6-316.2; P = .04) and mixed ROI-ML (150.8 mg/cm3; 95% CI, 7.2-294.5; P = .04). This difference remained significant and increased over time. Figure 5 shows the effect of RT on BMD in every ROI-ML and illustrates each ROI-ML responding differently to RT.
Fig. 5.
Mean bone mineral densities (in mg/cm3) of the lytic (A), blastic (B), and mixed (C) metastatic lesions (ROI-ML) for each lesion over time.
Discussion
The aim of this study was to determine the overall effect of palliative RT on bone mineral density in the proximal femur and metastatic lesions in patients with cancer and painful bone metastases. Additionally, we investigated whether these effects were different in femora with lytic, blastic, and mixed lesions.
In the proximal femora regions (ROI-PF), no differences in BMD were found between SF and MF RT. BMD decreased in ROI-PF that contained lytic lesions, increased in ROI-PF with blastic lesions, and did not change in ROI-PF with mixed lesions over time. After 10 weeks, differences in BMD in the radiation field (ROI-PF) were smaller than those in the metastatic lesions. This may indicate that the effect in ROI-PF was mainly due to BMD changes in the lesions (ROI-ML) and suggests that the irradiated femoral bone inside the radiation field but outside the lesion is unaffected or less affected by RT. A few studies found a smaller BMD increase in irradiated normal-appearing bone surrounding lytic metastases in vertebrae compared with the regions with vertebral lesions.10, 11 However, to our knowledge, the effect of RT on total bone volume in the radiation field has not been studied previously. Moreover, previous studies did not include blastic or mixed lesions.
When focusing on ROI-ML, BMD did not differ between SF and MF RT. Additionally, BMD in lytic ROI-ML did not change, which contradicts the findings in previous work.8, 9, 10, 11, 12 Koswig et al8 observed a decrease in BMD immediately after RT for both SF (1 × 8 Gy) and MF (10 × 3 Gy), followed by an increase in BMD after 6 months of 120% for SF and 173% for MF. After 10 weeks (comparable with our last time point), BMD increased approximately 106% and 137%, respectively. A similar response after MF (40 Gy) RT in vertebral lesions was observed by Reinbold et al10 who showed a decrease of 20% in BMD at the end of RT, followed by an increase of more than 60% after 3 months.10 The observation that RT ultimately increases BMD in lytic lesions is supported by other studies.9, 11 Actually, Koswig et al only observed significant differences in lesions that originated from breast cancer,8 which are known to be responsive to RT.30 When considering only metastases that arise from breast cancer in our study, no differences between SF and MF RT were seen. Hence, a beneficial effect of MF RT over SF RT on BMD was not observed in our study.
Our results, which contrast with those of previous studies, have several possible explanations. First, it should be noted that concurrent treatment with bisphosphonates may enhance the effect of RT on BMD.11, 12, 13 Although we did not find any interaction with medication, it may potentially have caused biased effects in the earlier studies that did not test this.8, 10 Second, the radiation doses in the previous studies were typically higher compared with the doses administered in our study. We administered doses of a total of 8 or 20 to 24 Gy, whereas other studies applied doses of up to 40 Gy.10, 11, 12 Third, we included metastatic lesions that originated from various primary tumors, some of which are known to be less responsive to RT than others.8, 30 In addition, most other studies included no or few femoral bone metastases but mostly vertebral and pelvic metastases, which are suggested to have a better BMD response to RT compared with metastases in the extremities.8, 30 Only one study included solely femoral metastases, and density changes in lesions were evaluated on the basis of x-ray test results with a reported response in 42% of patients. However, the study's follow-up ranged from 1 to 28 months, and higher response rates were found in patients who were followed for a longer period of time.12 The follow-up period in our study may have been too short to determine the long-term effects of RT on BMD. Finally, the most relevant difference between earlier studies and the current one is probably the detailed QCT-approach. We studied lesional volumes 3-dimensionally, which provided a more extensive analysis of RT effects on metastatic lesions. We performed several sensitivity analyses that proved that our 3-dimensional image registration was accurate. All previous research studied 2-dimensional ROI on the basis of x-ray test results12 or single CT scan image,8, 9, 10, 11 and temporal registration was accomplished by reproducing the patient position on the CT scanner8, 9, 10 or drawing ROI in each scan by hand.11 It can be questioned whether the same accuracy can be obtained with manual registration compared with our fully automated registration. The latter is not dependent on the arbitrary position of a limited number of landmarks but uses the overlap of a large number of voxels that are taken from the different images; therefore, they should be better than manual registration.28 For these reasons, we consider our study results to be reliable.
This study also has some limitations. As previously shown31, accurate identification of the margins of the actual bone lesions was difficult. Therefore, we added a rim of 6 mm around the edges to decrease the chance of omitting lesional tissue in the analysis, even though this may include nonaffected bone tissue. Also, categorization of lesions into pure lytic, pure blastic, and a mixed-type category using CT scans was complicated. Although the lesions were categorized in accordance with guidelines,32 some caution should be taken when interpreting differences between lesion types. Furthermore, the total number of patients included in the study was limited, and not all QCT scans were acquired for each patient. However, the main reasons for missing scans were death, fracture, and deteriorating condition, which is inevitable when analyzing data from patients with cancer who are in the palliative phase of their disease.
It is difficult to extrapolate BMD effect to femoral bone strength. Mean BMD did not change in lytic ROI-ML, which indicates that there was no progression of disease or remineralization of the bone tissue; hence, there was no change in bone strength. In contrast, BMD in blastic and mixed ROI-ML increased over time. However, the effect of these BMD increases on bone strength is difficult to interpret because denser blastic lesions could flag either disease progression or formation of new high-density bone tissue. Additionally, in mixed lesions, both blastic and lytic processes occur. These processes may cancel out a potential effect on bone mineral density. Therefore, the way changes in BMD affect femoral bone strength in blastic and mixed lesions should be investigated further.
Conclusions
In conclusion, higher total RT doses in patients with cancer and femoral metastases did not lead to significantly higher BMD up to 10 weeks after palliative RT, which brings into question the clinical relevance of MF over SF to stabilize femoral bone within this time period. Additionally, 10 weeks after RT, a significant increase in BMD was observed in blastic and mixed lesions but not in lytic lesions. Whether this implies progression or remineralization is unclear, especially since there was no control group of patients who received no RT. Also, the subsequent clinical effect of these changes on femoral bone strength remains unknown and needs to be investigated in the future.
Acknowledgments
The authors would like to thank Tom Knoop and Wouter Gevers for the development of the framework for registration and analysis, and the Department of Radiation Oncology in Nijmegen, RIF Leeuwarden and the Department of Clinical Oncology in Leiden for their help.
Footnotes
Sources of support: This project was funded by the Dutch Cancer Society (KUN 2012-5591) and the Dutch Science Foundation NWO-STW (NPG.06778).
Conflicts of interest: F. Eggermont and L. Derikx report grants from the Dutch Cancer Society (KUN 2012-5591) during the study. E. Tanck reports grants from the Dutch Science Foundation NWO-STW (NPG.06778) during the study.
Supplementary material related to this article can be found at http://dx.doi.org/10.1016/j.adro.2016.11.001.
Supplementary data
References
- 1.Coleman R.E. Skeletal complications of malignancy. Cancer. 1997;80:1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 2.Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 3.Healey J.H., Brown H.K. Complications of bone metastases: surgical management. Cancer. 2000;88:2940–2951. doi: 10.1002/1097-0142(20000615)88:12+<2940::aid-cncr10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 4.Chow E., Zeng L., Salvo N., Dennis K., Tsao M., Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol) 2012;24:112–124. doi: 10.1016/j.clon.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Lutz S., Berk L., Chang E. Palliative radiotherapy for bone metastases: An ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79:965–976. doi: 10.1016/j.ijrobp.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Bedard G., Hoskin P., Chow E. Overall response rates to radiation therapy for patients with painful uncomplicated bone metastases undergoing initial treatment and retreatment. Radiother Oncol. 2014;112:125–127. doi: 10.1016/j.radonc.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 7.van der Linden Y., Roos D., Lutz S., Fairchild A. International variations in radiotherapy fractionation for bone metastases: geographic borders define practice patterns? Clin Oncol (R Coll Radiol) 2009;21:655–658. doi: 10.1016/j.clon.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Koswig S., Budach V. [Remineralization and pain relief in bone metastases after different radiotherapy fractions (10 times 3 Gy vs. 1 time 8 Gy). A prospective study] Strahlenther Onkol. 1999;175:500–508. doi: 10.1007/s000660050061. [DOI] [PubMed] [Google Scholar]
- 9.Chow E., Holden L., Rubenstein J. Computed tomography (CT) evaluation of breast cancer patients with osteolytic bone metastases undergoing palliative radiotherapy–a feasibility study. Radiother Oncol. 2004;70:291–294. doi: 10.1016/j.radonc.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Reinbold W.D., Wannenmacher M., Hodapp N., Adler C.P. Osteodensitometry of vertebral metastases after radiotherapy using quantitative computed tomography. Skeletal Radiol. 1989;18:517–521. doi: 10.1007/BF00351751. [DOI] [PubMed] [Google Scholar]
- 11.Foerster R., Eisele C., Bruckner T. Bone density as a marker for local response to radiotherapy of spinal bone metastases in women with breast cancer: A retrospective analysis. Radiat Oncol. 2015;10:368. doi: 10.1186/s13014-015-0368-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harada H., Katagiri H., Kamata M. Radiological response and clinical outcome in patients with femoral bone metastases after radiotherapy. J Radiat Res. 2010;51:131–136. doi: 10.1269/jrr.09096. [DOI] [PubMed] [Google Scholar]
- 13.Groenen K.H., Pouw M.H., Hannink G. The effect of radiotherapy, and radiotherapy combined with bisphosphonates or RANK ligand inhibitors on bone quality in bone metastases. A systematic review. Radiother Oncol. 2016;119:194–201. doi: 10.1016/j.radonc.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Saarto T., Janes R., Tenhunen M., Kouri M. Palliative radiotherapy in the treatment of skeletal metastases. Eur J Pain. 2002;6:323–330. doi: 10.1016/s1090-3801(02)00028-9. [DOI] [PubMed] [Google Scholar]
- 15.Smith H.S. Painful osseous metastases. Pain Physician. 2011;14:E373–E403. [PubMed] [Google Scholar]
- 16.Van der Linden Y.M., Dijkstra P.D., Kroon H.M. Comparative analysis of risk factors for pathological fracture with femoral metastases. J Bone Joint Surg Br. 2004;86:566–573. [PubMed] [Google Scholar]
- 17.Van der Linden Y.M., Kroon H.M., Dijkstra S.P. Simple radiographic parameter predicts fracturing in metastatic femoral bone lesions: results from a randomised trial. Radiother Oncol. 2003;69:21–31. doi: 10.1016/s0167-8140(03)00232-9. [DOI] [PubMed] [Google Scholar]
- 18.Steenland E., Leer J.W., van Houwelingen H. The effect of a single fraction compared to multiple fractions on painful bone metastases: A global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52:101–109. doi: 10.1016/s0167-8140(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 19.Toma C.D., Dominkus M., Nedelcu T. Metastatic bone disease: A 36-year single centre trend-analysis of patients admitted to a tertiary orthopaedic surgical department. J Surg Oncol. 2007;96:404–410. doi: 10.1002/jso.20787. [DOI] [PubMed] [Google Scholar]
- 20.Derikx L.C., van Aken J.B., Janssen D. The assessment of the risk of fracture in femora with metastatic lesions: comparing case-specific finite element analyses with predictions by clinical experts. Journal of Bone & Joint Surgery - British Volume. 2012;94(8):1135–1142. doi: 10.1302/0301-620X.94B8.28449. [DOI] [PubMed] [Google Scholar]
- 21.Derikx LC, Groenen K, van Bon GA, et al. Patient-specific finite element models discriminate between patients with and without a pathological fracture in metastatic bone disease. 57th Annual Meeting of the Orthopaedic Research Society. Long Beach, California, United States, 2011.
- 22.Fairbank J.C., Couper J., Davies J.B., O'Brien J.P. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 23.Stel V.S., Smit J.H., Pluijm S.M., Visser M., Deeg D.J., Lips P. Comparison of the LASA Physical Activity Questionnaire with a 7-day diary and pedometer. J Clin Epidemiol. 2004;57:252–258. doi: 10.1016/j.jclinepi.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Ware J.E., Jr., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 25.Bellamy N., Buchanan W.W., Goldsmith C.H., Campbell J., Stitt L.W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 26.Klein S., Staring M., Murphy K., Viergever M.A., Pluim J.P.W. Elastix: A toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2010;29:196–205. doi: 10.1109/TMI.2009.2035616. [DOI] [PubMed] [Google Scholar]
- 27.Shamonin D.P., Bron E.E., Lelieveldt B.P.F. Fast parallel image registration on CPU and GPU for diagnostic classification of Alzheimer's disease. Front Neuroinform. 2014;7:50. doi: 10.3389/fninf.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knoop T.H., Derikx L.C., Verdonschot N., Slump C.H. A novel framework for the temporal analysis of bone mineral density in metastatic lesions using CT images of the femur. SPIE Medical Imaging: International Society for Optics and Photonics. 2015 94143A-43A-11. [Google Scholar]
- 29.Budtz-Jorgensen E., Keiding N., Grandjean P., Weihe P. Confounder selection in environmental epidemiology: Assessment of health effects of prenatal mercury exposure. Ann Epidemiol. 2007;17:27–35. doi: 10.1016/j.annepidem.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Rieden K., Adolph J., Lellig U., zum Winkel K. [The radiotherapeutic effect on bone metastases in relation to the frequency of metastases, sites of metastases and histology of the primary tumor] Strahlenther Onkol. 1989;165:380–385. [PubMed] [Google Scholar]
- 31.Dijkstra P.D., Oudkerk M., Wiggers T. Prediction of pathological subtrochanteric fractures due to metastatic lesions. Arch Orthop Trauma Surg. 1997;116:221–224. doi: 10.1007/BF00393714. [DOI] [PubMed] [Google Scholar]
- 32.Mulder J.D., Kroon H.M., Schutte H.E., Taconis W.K. The diagnosis of bone tumours. In: Mulder J.D., Kroon H.M., Schutte H.E., editors. Radiologic atlas of bone tumours. Elsevier; Amsterdam: 1993. pp. 28–31. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.